comproportionation on:
[Wikipedia]
[Google]
[Amazon]
Comproportionation or symproportionation is a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number. It is the opposite of disproportionation.Shriver, D. F.; Atkins, P. W.; Overton, T. L.; Rourke, J. P.; Weller, M. T.; Armstrong, F. A. (2006). “Inorganic Chemistry” W. H. Freeman, New York. .
 In
In
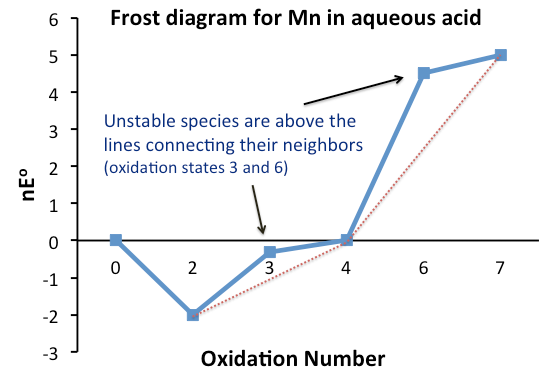
Frost diagrams
 In
In electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
, the tendency of two redox species to disproportionate, or comproportionate, can be determined by examining their Frost diagram. It is a graphical plot of as a function of the oxidation number for the different redox species of a given element.
The Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
Δ''G''° is related to the reduction potential ''E''° by the formula: or , where ''n'' is the number of transferred electrons, and ''F'' is the Faraday constant
In physical chemistry, the Faraday constant (symbol , sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge () by the amount () of elementary charge carriers in any given sample of matter: it ...
).
If the value of for a species is lower than the line joining two adjacent, or more generally, neighboring species, having thus a lower and a higher oxidation number, then this species is more stable than its neighbors, and the two surrounding species will undergo comproportionation to minimize the Gibbs free energy of the system. Example: a mixture of Mn(III) and Mn(VI) will comproportionate towards Mn(IV) as illustrated in the Frost diagram for manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
. Non-adjacent neighboring species of Mn obeying the same general rule will also react together as, e.g., and to form . So, the more distant Mn(II) and Mn(VII) can also react together to form Mn(IV). The reacting redox species do not have to be necessarily adjacent on a Frost diagram.
The comproportionation reaction cannot easily occur in solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
s in which the potentially reactive species are immobile and thus cannot react together, or the reaction will be extremely slow and will also require high temperature close to the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
of the solid to render the reactive species more mobile. However, if these species are soluble, and thus highly mobile in an aqueous solution, they will much more easily encounter, react and undergo comproportionation. In the case of heterogeneous systems involving a solution and one or more solid phases, as in a lead–acid battery
The lead–acid battery is a type of rechargeable battery first invented in 1859 by French physicist Gaston Planté. It was the first type of rechargeable battery to be invented. Compared to modern rechargeable batteries, lead–acid batteries ha ...
, a comproportionation reaction is possible thanks to the mobile dissolved Pb ions released into solution at the surface of the battery solid electrodes ( Pb and PbO2). In the gas phase, the comproportionation reaction is much faster because of the much higher mobility of the reacting species as illustrated, e.g., in the Claus reaction where and react together to form elemental sulfur. Various classical comproportionation reactions are detailed in the series of examples here below.
Examples
* In lead batteries, the spontaneous reaction is: :: Pb + PbO2 + 2 H2SO4 → 2 PbSO4 + 2 H2O * The laboratory preparation of manganese dioxide involves comproportionation of Mn(II) and Mn(VII) reagents: :: 2 + 3 + 2 → 5 + + 2 * Inselenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
chemistry:
:: 15 Se + SeCl4 + 4 AlCl3 → 2 Se8 lCl4sub>2
* In the Claus process, two gaseous compounds of sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
comproportionate in the presence of a catalyst to give elemental sulfur:
:: 2 H2S + SO2 → 3 S + 2 H2O
* In halogen chemistry:
:: IO3− + 5 I− + 6 H + → 3 I2 + 3 H2O
* In anammox (anaerobic ammonium oxidation) biochemistry:
:: NH4+ + NO2− → N2 + 2 H2O
* Iron(III) chloride reacts with iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
powder to form iron(II) chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water ...
:
::
References
{{Reflist Chemical reactions