Carbon Steel on:
[Wikipedia]
[Google]
[Amazon]
Carbon steel is a steel with
/ref> making it malleable and ductile. Mild steel has a relatively low tensile strength, but it is cheap and easy to form. Surface hardness can be increased with carburization. In applications where large cross-sections are used to minimize deflection, failure by yield is not a risk so low-carbon steels are the best choice, for example as structural steel. The density of mild steel is approximately 7.85 g/cm3 (7850 kg/m3 or 0.284 lb/in3) and the
 The purpose of heat treating carbon steel is to change the mechanical properties of steel, usually ductility, hardness, yield strength, or impact resistance. Note that the electrical and thermal conductivity are only slightly altered. As with most strengthening techniques for steel,
The purpose of heat treating carbon steel is to change the mechanical properties of steel, usually ductility, hardness, yield strength, or impact resistance. Note that the electrical and thermal conductivity are only slightly altered. As with most strengthening techniques for steel,
carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute
The American Iron and Steel Institute is an association of North American steel producers. With its predecessor organizations, is one of the oldest trade associations in the United States, dating back to 1855. It assumed its present form in 1908 ...
(AISI) states:
* no minimum content is specified or required for chromium, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, p ...
, molybdenum, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
, niobium, titanium
Titanium is a chemical element with the Symbol (chemistry), symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resista ...
, tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
, vanadium, zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
, or any other element to be added to obtain a desired alloying effect;
* the specified minimum for copper does not exceed 0.40%;
* or the maximum content specified for any of the following elements does not exceed the percentages noted: manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
1.65%; silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
0.60%; copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
0.60%.
The term ''carbon steel'' may also be used in reference to steel which is not stainless steel; in this use carbon steel may include alloy steel
Alloy steel is steel that is alloyed with a variety of elements in total amounts between 1.0% and 50% by weight to improve its mechanical properties. Alloy steels are broken down into two groups: low alloy steels and high alloy steels. The differe ...
s. High carbon steel has many different uses such as milling machines, cutting tools (such as chisels) and high strength wires. These applications require a much finer microstructure, which improves the toughness.
Carbon steel is a popular metal choice for knife-making due to its high amount of carbon, giving the blade more edge retention. To make the most out of this type of steel it is very important to heat treat it properly. If not, the knife may end up being brittle, or too soft to hold an edge.
As the carbon percentage content rises, steel has the ability to become harder and stronger through heat treating
Heat treating (or heat treatment) is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are als ...
; however, it becomes less ductile
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
. Regardless of the heat treatment, a higher carbon content reduces weldability The weldability, also known as joinability,. of a material refers to its ability to be welded. Many metals and thermoplastics can be welded, but some are easier to weld than others (see Rheological weldability). A material's weldability is used to ...
. In carbon steels, the higher carbon content lowers the melting point.
Type
Mild or low-carbon steel
Mild steel (iron containing a small percentage of carbon, strong and tough but not readily tempered), also known as plain-carbon steel and low-carbon steel, is now the most common form of steel because its price is relatively low while it provides material properties that are acceptable for many applications. Mild steel contains approximately 0.05–0.30% carbon"Classification of Carbon and Low-Alloy Steels"/ref> making it malleable and ductile. Mild steel has a relatively low tensile strength, but it is cheap and easy to form. Surface hardness can be increased with carburization. In applications where large cross-sections are used to minimize deflection, failure by yield is not a risk so low-carbon steels are the best choice, for example as structural steel. The density of mild steel is approximately 7.85 g/cm3 (7850 kg/m3 or 0.284 lb/in3) and the
Young's modulus
Young's modulus E, the Young modulus, or the modulus of elasticity in tension or compression (i.e., negative tension), is a mechanical property that measures the tensile or compressive stiffness of a solid material when the force is applied le ...
is .
Low-carbon steels display ''yield-point runout'' where the material has two yield point
In materials science and engineering, the yield point is the point on a stress-strain curve that indicates the limit of elastic behavior and the beginning of plastic behavior. Below the yield point, a material will deform elastically and wi ...
s. The first yield point (or upper yield point) is higher than the second and the yield drops dramatically after the upper yield point. If a low-carbon steel is only stressed to some point between the upper and lower yield point then the surface develops Lüder bands
Lüder is a municipality in the district of Uelzen, in Lower Saxony, Germany.
Through the area flows a small river of the same name, with its source in the upland bog of the Völzberger Köpfchen and its mouth where it flows into the Fulda
...
. Low-carbon steels contain less carbon than other steels and are easier to cold-form, making them easier to handle. Typical applications of low carbon steel are car parts, pipes, construction, and food cans.
High-tensile steel
High-tensile steels are low-carbon, or steels at the lower end of the medium-carbon range, which have additional alloying ingredients in order to increase their strength, wear properties or specificallytensile strength
Ultimate tensile strength (UTS), often shortened to tensile strength (TS), ultimate strength, or F_\text within equations, is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials t ...
. These alloying ingredients include chromium, molybdenum, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ta ...
, manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow ...
, and vanadium. Impurities such as phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
and sulfur have their maximum allowable content restricted.
* 41xx steel
41xx steel is a family of SAE steel grades, as specified by the Society of Automotive Engineers (SAE). Alloying elements include chromium and molybdenum, and as a result these materials are often informally referred to as chromoly steel (common v ...
** 4140 steel
** 4145 steel
* 4340 steel
** 300M steel
* EN25 steel – 2.521% nickel-chromium-molybdenum steel
* EN26 steel
Higher-carbon steels
Carbon steels which can successfully undergo heat-treatment have a carbon content in the range of 0.30–1.70% by weight. Trace impurities of various other elements can significantly affect the quality of the resulting steel. Trace amounts of sulfur in particular make the steel red-short, that is, brittle and crumbly at working temperatures. Low-alloy carbon steel, such as A36 grade, contains about 0.05% sulfur and melt around .Manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy use ...
is often added to improve the hardenability
The hardenability of a metal alloy is the depth to which a material is hardened after putting it through a heat treatment process. It should not be confused with hardness, which is a measure of a sample's resistance to indentation or scratching. I ...
of low-carbon steels. These additions turn the material into a low-alloy steel by some definitions, but AISI's definition of carbon steel allows up to 1.65% manganese by weight.
There are two types of higher carbon steels which are high carbon steel and the ultra high carbon steel. The reason for the limited use of high carbon steel is that it has extremely poor ductility and weldability and has a higher cost of production. the applications best suited for the high carbon steels is its use in the spring industry, farm industry, and in the production of wide range of high-strength wires.
AISI classification
Carbon steel is broken down into four classes based on carbon content:Low-carbon steel
0.05 to 0.15% carbon (plain carbon steel) content.Medium-carbon steel
Approximately 0.3–0.5% carbon content. Balances ductility and strength and has good wear resistance; used for large parts, forging and automotive components.High-carbon steel
Approximately 0.6 to 1.0% carbon content. Very strong, used for springs, edged tools, and high-strength wires.Ultra-high-carbon steel
Approximately 1.25–2.0% carbon content. Steels that can be tempered to great hardness. Used for special purposes like (non-industrial-purpose) knives, axles, and punches. Most steels with more than 2.5% carbon content are made usingpowder metallurgy
Powder metallurgy (PM) is a term covering a wide range of ways in which materials or components are made from metal powders. PM processes can reduce or eliminate the need for subtractive processes in manufacturing, lowering material losses and ...
.
Heat treatment
 The purpose of heat treating carbon steel is to change the mechanical properties of steel, usually ductility, hardness, yield strength, or impact resistance. Note that the electrical and thermal conductivity are only slightly altered. As with most strengthening techniques for steel,
The purpose of heat treating carbon steel is to change the mechanical properties of steel, usually ductility, hardness, yield strength, or impact resistance. Note that the electrical and thermal conductivity are only slightly altered. As with most strengthening techniques for steel, Young's modulus
Young's modulus E, the Young modulus, or the modulus of elasticity in tension or compression (i.e., negative tension), is a mechanical property that measures the tensile or compressive stiffness of a solid material when the force is applied le ...
(elasticity) is unaffected. All treatments of steel trade ductility for increased strength and vice versa. Iron has a higher solubility for carbon in the austenite phase; therefore all heat treatments, except spheroidizing and process annealing, start by heating the steel to a temperature at which the austenitic phase can exist. The steel is then quenched (heat drawn out) at a moderate to low rate allowing carbon to diffuse out of the austenite forming iron-carbide (cementite) and leaving ferrite, or at a high rate, trapping the carbon within the iron thus forming martensite. The rate at which the steel is cooled through the eutectoid
A eutectic system or eutectic mixture ( ) is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the ''eutectic tempe ...
temperature (about ) affects the rate at which carbon diffuses out of austenite and forms cementite. Generally speaking, cooling swiftly will leave iron carbide finely dispersed and produce a fine grained pearlite
Pearlite is a two-phased, lamellar (or layered) structure composed of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. During slow cooling of an iron-carbon alloy, pearlite form ...
and cooling slowly will give a coarser pearlite. Cooling a hypoeutectoid steel (less than 0.77 wt% C) results in a lamellar-pearlitic structure of iron carbide layers with α- ferrite (nearly pure iron) between. If it is hypereutectoid steel (more than 0.77 wt% C) then the structure is full pearlite with small grains (larger than the pearlite lamella) of cementite
Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, bri ...
formed on the grain boundaries. A eutectoid steel (0.77% carbon) will have a pearlite structure throughout the grains with no cementite at the boundaries. The relative amounts of constituents are found using the lever rule. The following is a list of the types of heat treatments possible:
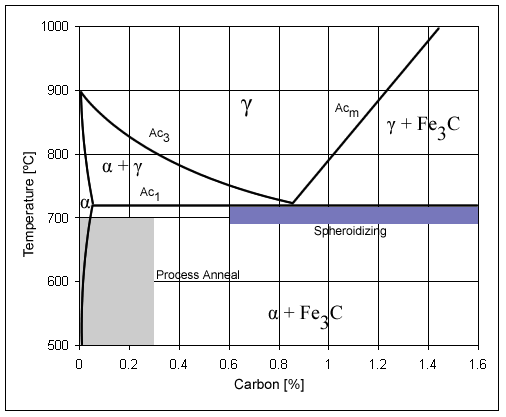
; Spheroidizing: Spheroidite forms when carbon steel is heated to approximately for over 30 hours. Spheroidite can form at lower temperatures but the time needed drastically increases, as this is a diffusion-controlled process. The result is a structure of rods or spheres of cementite within primary structure (ferrite or pearlite, depending on which side of the eutectoid you are on). The purpose is to soften higher carbon steels and allow more formability. This is the softest and most ductile form of steel.
; Full annealing: Carbon steel is heated to approximately for 1 hour; this ensures all the ferrite transforms into austenite (although cementite
Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, bri ...
might still exist if the carbon content is greater than the eutectoid). The steel must then be cooled slowly, in the realm of per hour. Usually it is just furnace cooled, where the furnace is turned off with the steel still inside. This results in a coarse pearlitic structure, which means the "bands" of pearlite
Pearlite is a two-phased, lamellar (or layered) structure composed of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. During slow cooling of an iron-carbon alloy, pearlite form ...
are thick. Fully annealed steel is soft and ductile
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
, with no internal stresses, which is often necessary for cost-effective forming. Only spheroidized steel is softer and more ductile.
; Process annealing: A process used to relieve stress in a cold-worked carbon steel with less than 0.3% C. The steel is usually heated to for 1 hour, but sometimes temperatures as high as . The image rightward shows the process annealing area.
; Isothermal annealing: It is a process in which hypoeutectoid steel is heated above the upper critical temperature. This temperature is maintained for a time and then reduced to below the lower critical temperature and is again maintained. It is then cooled to room temperature. This method eliminates any temperature gradient.
; Normalizing: Carbon steel is heated to approximately for 1 hour; this ensures the steel completely transforms to austenite. The steel is then air-cooled, which is a cooling rate of approximately per minute. This results in a fine pearlitic structure, and a more-uniform structure. Normalized steel has a higher strength than annealed steel; it has a relatively high strength and hardness.
; Quenching
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as pha ...
: Carbon steel with at least 0.4 wt% C is heated to normalizing temperatures and then rapidly cooled (quenched) in water, brine, or oil to the critical temperature. The critical temperature is dependent on the carbon content, but as a general rule is lower as the carbon content increases. This results in a martensitic structure; a form of steel that possesses a super-saturated carbon content in a deformed body-centered cubic (BCC) crystalline structure, properly termed body-centered tetragonal (BCT), with much internal stress. Thus quenched steel is extremely hard but brittle
A material is brittle if, when subjected to stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength. Br ...
, usually too brittle for practical purposes. These internal stresses may cause stress cracks on the surface. Quenched steel is approximately three times harder (four with more carbon) than normalized steel.
; Martempering (marquenching): Martempering is not actually a tempering procedure, hence the term ''marquenching''. It is a form of isothermal heat treatment applied after an initial quench, typically in a molten salt bath, at a temperature just above the "martensite start temperature". At this temperature, residual stresses within the material are relieved and some bainite may be formed from the retained austenite which did not have time to transform into anything else. In industry, this is a process used to control the ductility and hardness of a material. With longer marquenching, the ductility increases with a minimal loss in strength; the steel is held in this solution until the inner and outer temperatures of the part equalize. Then the steel is cooled at a moderate speed to keep the temperature gradient minimal. Not only does this process reduce internal stresses and stress cracks, but it also increases impact resistance.
; Tempering: This is the most common heat treatment encountered because the final properties can be precisely determined by the temperature and time of the tempering. Tempering involves reheating quenched steel to a temperature below the eutectoid
A eutectic system or eutectic mixture ( ) is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the ''eutectic tempe ...
temperature and then cooling. The elevated temperature allows very small amounts spheroidite to form, which restores ductility but reduces hardness. Actual temperatures and times are carefully chosen for each composition.
; Austempering: The austempering process is the same as martempering, except the quench is interrupted and the steel is held in the molten salt bath at temperatures between , and then cooled at a moderate rate. The resulting steel, called bainite, produces an acicular microstructure in the steel that has great strength (but less than martensite), greater ductility, higher impact resistance, and less distortion than martensite steel. The disadvantage of austempering is it can be used only on a few sheets of steel, and it requires a special salt bath.Smith, p. 391
Case hardening
Case hardening processes harden only the exterior of the steel part, creating a hard, wear-resistant skin (the "case") but preserving a tough and ductile interior. Carbon steels are not very hardenable meaning they can not be hardened throughout thick sections. Alloy steels have a better hardenability, so they can be through-hardened and do not require case hardening. This property of carbon steel can be beneficial, because it gives the surface good wear characteristics but leaves the core flexible and shock-absorbing.Forging temperature of steel
See also
*Aermet AerMet alloy is an ultra-high strength type of martensitic alloy steel. The main alloying elements are cobalt and nickel, but chromium, molybdenum and carbon are also added. Its exceptional properties are hardness, tensile strength, fracture toughn ...
*Cold working
In metallurgy, cold forming or cold working is any metalworking process in which metal is shaped below its recrystallization temperature, usually at the ambient temperature. Such processes are contrasted with hot working techniques like hot r ...
*Eglin steel Eglin steel (ES-1) is a high- strength, high-performance, low-alloy, low-cost steel, developed for a new generation of bunker buster type bombs, e.g. the Massive Ordnance Penetrator and the improved version of the GBU-28 bomb known as EGBU-28. It ...
(a low-cost precipitation-hardened high-strength steel)
* Forging
* Hot working
*Maraging steel
Maraging steels (a portmanteau of " martensitic" and "aging") are steels that are known for possessing superior strength and toughness without losing ductility. ''Aging'' refers to the extended heat-treatment process. These steels are a special cla ...
(precipitation-hardened high-strength steels)
*Welding
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as bra ...
(high-strength steels)
References
Bibliography
* * * {{Authority control Steels Metallurgical processes