carbometalation on:
[Wikipedia]
[Google]
[Amazon]
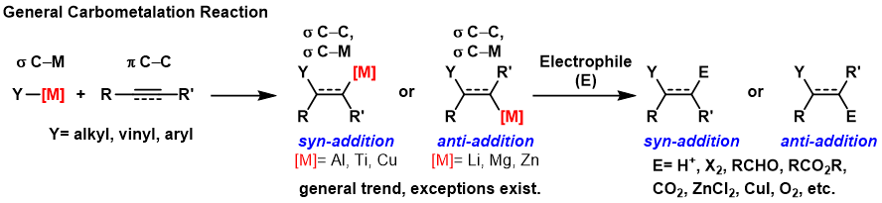 A carbometalation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions (oligomerization or polymerization see Ziegler-Natta polymerization) or it can be reacted with a variety of
A carbometalation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions (oligomerization or polymerization see Ziegler-Natta polymerization) or it can be reacted with a variety of
 The most common trialkyl aluminum reagents for this transformation are
The most common trialkyl aluminum reagents for this transformation are 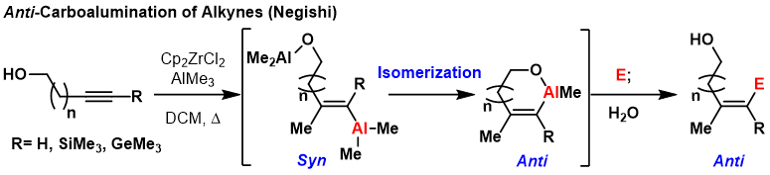 This forms a thermodynamically favorable
This forms a thermodynamically favorable  The carboalumination of alkenes to form substituted alkanes can be rendered enantioselective if prochiral alkenes are used. In these reactions, a
The carboalumination of alkenes to form substituted alkanes can be rendered enantioselective if prochiral alkenes are used. In these reactions, a
 Carbolithiation is the addition of an
Carbolithiation is the addition of an
 A carbometalation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions (oligomerization or polymerization see Ziegler-Natta polymerization) or it can be reacted with a variety of
A carbometalation is any reaction where a carbon-metal bond reacts with a carbon-carbon π-bond to produce a new carbon-carbon σ-bond and a carbon-metal σ-bond. The resulting carbon-metal bond can undergo further carbometallation reactions (oligomerization or polymerization see Ziegler-Natta polymerization) or it can be reacted with a variety of electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
s including halogenating reagents, carbonyls
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
, oxygen, and inorganic salts to produce different organometallic reagents. Carbometalations can be performed on alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s and alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic ...
s to form products with high geometric purity or enantioselectivity
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
, respectively. Some metals prefer to give the ''anti''-addition product with high selectivity and some yield the syn-addition product. The outcome of ''syn'' and ''anti''- addition products is determined by the mechanism of the carbometalation.
Carboalumination
The carboalumination reaction is most commonly catalyzed byzirconocene dichloride
Zirconocene dichloride is an organozirconium compound composed of a zirconium central atom, with two cyclopentadienyl and two chloro ligands. It is a colourless diamagnetic solid that is somewhat stable in air.
Preparation and structure
Zirconoce ...
(or related catalyst). Some carboaluminations are performed with titanocene
Titanocene dichloride is the organotitanium compound with the formula ( ''η''5-C5H5)2TiCl2, commonly abbreviated as Cp2TiCl2. This metallocene is a common reagent in organometallic and organic synthesis. It exists as a bright red solid that slowl ...
complexes. This reaction is sometimes referred to as the Zr- catalyzed asymmetric carboalumination of alkenes (ZACA) or the Zr-catalyzed methylalumination of alkynes (ZMA).
 The most common trialkyl aluminum reagents for this transformation are
The most common trialkyl aluminum reagents for this transformation are trimethylaluminium
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industriall ...
, triethylaluminium
Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( C2H5)6 (abbreviated as Al2Et6 or TEA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially ...
, and sometimes triisobutylaluminium. When using trialkylaluminum reagents that have beta-hydrides, eliminations and hydroaluminum reactions become competing processes. The general mechanism of the ZMA reaction can be described as first the formation of the active catalytic species from the pre-catalyst zirconocene dichloride through its reaction with trimethyl aluminum. First transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
of a methyl from the aluminum to the zirconium occurs. Next, chloride abstraction by aluminum creates a cationic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
zirconium species that is closely associated with an anionic aluminum complex. This zirconium cation can coordinate an alkene or alkyne where migratory insertion
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanis ...
of a methyl then takes place. The resultant vinyl or alkyl zirconium species can undergo a reversible, but stereoretentive transmetalation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
with an organoaluminum to provide the carboalumination product and regeneration of the zirconcene dichloride catalyst. This process generally provides the syn-addition product; however, conditions exist to provide the anti-addition product though a modified mechanism.
Trimethyl Silyl ( TMS) protected alkynes, trimethyl germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is lustrous, hard-brittle, grayish-white and similar in appearance to silicon. It is a metalloid in the carbon group that is chemically similar to its group neighbors ...
alkynes, and terminal alkynes can produce anti-carboalumination products at room temperature or elevated temperatures if a coordinating group is nearby on the substrate. In these reactions, first syn-carboalumination takes place under the previously outlined mechanism. Then, another equivalent of aluminum that is coordinated to the directing group can displace the vinyl aluminum, inverting the geometry at the carbon where displacement takes place.
 This forms a thermodynamically favorable
This forms a thermodynamically favorable metallacycle
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates ...
to prevent subsequent inversions. Formally, this process provides anti-carboalumination products that can be quenched with electrophiles. A limitation of this methodology is that the directing group must be sufficiently close to the carbon-carbon π-bond to form a thermodynamically favorable ring or else mixtures of geometric isomers
Geometry (; ) is, with arithmetic, one of the oldest branches of mathematics. It is concerned with properties of space such as the distance, shape, size, and relative position of figures. A mathematician who works in the field of geometry is ca ...
will form.
 The carboalumination of alkenes to form substituted alkanes can be rendered enantioselective if prochiral alkenes are used. In these reactions, a
The carboalumination of alkenes to form substituted alkanes can be rendered enantioselective if prochiral alkenes are used. In these reactions, a chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
indenyl zirconium catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
is used to induce enantioselectivity. In these reactions, high enantioselectivities were obtained for several trialkyl aluminum reagents, however, the yield decreases dramatically with each additional carbon of the alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
chain on the trialkyl aluminum reagent.
Carbolithiation
 Carbolithiation is the addition of an
Carbolithiation is the addition of an organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
across a carbon-carbon pi-bond. The organolithium reagents used in this transformation can be commercial (such as n-butyllithium
''n''-Butyllithium C4H9Li (abbreviated ''n''-BuLi) is an organolithium reagent. It is widely used as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). Also, it is broadly emp ...
) or can be generated through deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
or lithium halogen exchange. Both inter- and intramolecular examples of carbolithiation exist and can be used in synthesis to generate complexity. Organolithiums are highly reactive chemicals and often the resulting organolithium reagent generated from the carbolithiation can continue to react with electrophiles or remaining starting material (resulting in polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many f ...
). This reaction has been rendered enantioselective through the use of sparteine
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in '' Lupinus mutabilis'', and is thought to chelate the bivalent cations calcium and ...
, which can chelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
the lithium ion and induce chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
. Today, this is not a common strategy due to a shortage of natural sparteine. However, recent advances in the synthesis of sparteine surrogates and their effective application in carbolithiation have reactivated interest in this strategy.
Another demonstration of this reaction type is an alternative route to tamoxifen
Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and treat breast cancer in women and men. It is also being studied for other types of cancer. It has b ...
starting from diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a ligand in organometallic chemistry.
Prepar ...
and ethyllithium: The capturing electrophile here is triisopropyl borate forming the boronic acid
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, membe ...
R–B(OH)2. The second step completing tamoxifen is a Suzuki reaction
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium, palladium(0) complex. It was first published in 1979 by Akira ...
.
:
As a consequence of the high reactivity of organolithiums as strong bases and strong nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they ar ...
s, the substrate scope of the carbolithiation is generally limited to chemicals that do not contain acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a se ...
ic or electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the r ...
s.
Carbomagnesiation and carbozincation
Due to the decreasednucleophilicity
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
of Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . ...
s (organomagnesium) and organozinc
Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.The Chemistry of Organozinc Compou ...
reagents, non-catalyzed carbomagnesiation and carbozincation reactions are typically only observed on activated or strained alkenes and alkynes. For example, electron withdrawing
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
groups like esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
, nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including me ...
s or sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s must be in conjugation with the carbon-carbon π-system (see Michael reaction) or a directing group like an alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
or amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
must be nearby to direct the reaction. These reactions can be catalyzed by a variety of transition metals such as iron, copper, zirconium, nickel, cobalt and others.
Illustrative is the Fe-catalyzed reaction of methylphenylacetylene with phenylmagnesium bromide
Phenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF). Phenylmagnesium bromide is a Grignard reagent. It is ...
, which generates a vinyl magnesium intermediate. Hydrolysis affords the diphenylalkene:
:
Carbopalladation
Carbopalladations can be a description of the elementary step of a reaction catalyzed by a palladium catalyst (Mizoroki-Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a sub ...
) and can also refer to a carbometalation reaction with a palladium catalyst ( alkene difunctionalization, hydrofunctionalization A hydrofunctionalization reaction is the addition of hydrogen and another univalent fragment (X) across a carbon-carbon or carbon-heteroatom multiple bond. Often, the term ''hydrofunctionalization'' without modifier refers specifically to the use o ...
, or reductive heck)
References
{{Organometallics Organic reactions Organometallic chemistry