Boron Nitride on:
[Wikipedia]
[Google]
[Amazon]
Boron nitride is a thermally and chemically resistant
File:Boron-nitride-(hexagonal)-side-3D-balls.png, Hexagonal form (h-BN)
hexagonal analogous to
analogous to
wurtzite structure
analogous to lonsdaleite
National Pollutant Inventory: Boron and Compounds
at University of Oxford {{DEFAULTSORT:Boron Nitride Boron compounds Ceramic materials Nitrides III-V semiconductors Non-petroleum based lubricants Dry lubricants Abrasives Superhard materials Neutron poisons Monolayers III-V compounds Boron–nitrogen compounds Zincblende crystal structure Wurtzite structure type
refractory
In materials science, a refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase, ...
compound of boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has t ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
BN. It exists in various crystalline forms that are isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
to a similarly structured carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
lattice. The hexagonal form corresponding to graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, b ...
is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.
Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology.
Structure
Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to varying bulk properties of the material.Amorphous form (a-BN)
The amorphous form of boron nitride (a-BN) is non-crystalline, lacking any long-distance regularity in the arrangement of its atoms. It is analogous to amorphous carbon. All other forms of boron nitride are crystalline.Hexagonal form (h-BN)
The most stable crystalline form is the hexagonal one, also called h-BN, α-BN, g-BN, and ''graphitic boron nitride''. Hexagonal boron nitride (point group = D6h; space group = P63/mmc) has a layered structure similar to graphite. Within each layer, boron and nitrogen atoms are bound by strongcovalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between ato ...
s, whereas the layers are held together by weak van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and ...
s. The interlayer "registry" of these sheets differs, however, from the pattern seen for graphite, because the atoms are eclipsed, with boron atoms lying over and above nitrogen atoms. This registry reflects the local polarity of the B–N bonds, as well as interlayer N-donor/B-acceptor characteristics. Likewise, many metastable forms consisting of differently stacked polytypes exist. Therefore, h-BN and graphite are very close neighbors, and the material can accommodate carbon as a substituent element to form BNCs. BC6N hybrids have been synthesized, where carbon substitutes for some B and N atoms.
Cubic form (c-BN)
Cubic boron nitride has a crystal structure analogous to that ofdiamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, b ...
. Consistent with diamond being less stable than graphite, the cubic form is less stable than the hexagonal form, but the conversion rate between the two is negligible at room temperature, as it is for diamond. The cubic form has the sphalerite crystal structure, the same as that of diamond (with ordered B and N atoms), and is also called β-BN or c-BN.
Wurtzite form (w-BN)
The wurtzite form of boron nitride (w-BN; point group = C6v; space group = P63mc) has the same structure as lonsdaleite, a rare hexagonal polymorph of carbon. As in the cubic form, the boron and nitrogen atoms are grouped intotetrahedra
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all th ...
. In the wurtzite form, the boron and nitrogen atoms are grouped into 6-membered rings. In the cubic form all rings are in the chair configuration, whereas in w-BN the rings between 'layers' are in boat configuration. Earlier optimistic reports predicted that the wurtzite form was very strong, and was estimated by a simulation as potentially having a strength 18% stronger than that of diamond. Since only small amounts of the mineral exist in nature, this has not yet been experimentally verified. Its hardness is 46 GPa, slightly harder than commercial borides but softer than the cubic form of boron nitride.
hexagonal analogous to
graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
File:Boron-nitride-(sphalerite)-3D-balls.png, Cubic form (c-BN)sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-V ...
structureanalogous to
diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, b ...
File:Boron-nitride-(wurtzite)-3D-balls.png, Wurtzite form (w-BN)wurtzite structure
analogous to lonsdaleite
Properties
Physical
The partly ionic structure of BN layers in h-BN reduces covalency and electrical conductivity, whereas the interlayer interaction increases resulting in higher hardness of h-BN relative to graphite. The reduced electron-delocalization in hexagonal-BN is also indicated by its absence of color and a large band gap. Very different bonding – strong covalent within the basal planes (planes where boron and nitrogen atoms are covalently bonded) and weak between them – causes high anisotropy of most properties of h-BN. For example, the hardness, electrical and thermal conductivity are much higher within the planes than perpendicular to them. On the contrary, the properties of c-BN and w-BN are more homogeneous and isotropic. Those materials are extremely hard, with the hardness of bulk c-BN being slightly smaller and w-BN even higher than that of diamond. Polycrystalline c-BN with grain sizes on the order of 10 nm is also reported to haveVickers hardness
The Vickers hardness test was developed in 1921 by Robert L. Smith and George E. Sandland at Vickers Ltd as an alternative to the Brinell method to measure the hardness of materials. The Vickers test is often easier to use than other hardness ...
comparable or higher than diamond. Because of much better stability to heat and transition metals, c-BN surpasses diamond in mechanical applications, such as machining steel. The thermal conductivity of BN is among the highest of all electric insulators (see table).
Boron nitride can be doped p-type with beryllium and n-type with boron, sulfur, silicon or if co-doped with carbon and nitrogen. Both hexagonal and cubic BN are wide-gap semiconductors with a band-gap energy corresponding to the UV region. If voltage is applied to h-BN or c-BN, then it emits UV light in the range 215–250 nm and therefore can potentially be used as light-emitting diode
A light-emitting diode (LED) is a semiconductor Electronics, device that Light#Light sources, emits light when Electric current, current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy i ...
s (LEDs) or lasers.
Little is known on melting behavior of boron nitride. It sublimates at 2973 °C at normal pressure releasing nitrogen gas and boron, but melts at elevated pressure.
Thermal stability
Hexagonal and cubic BN (and probably w-BN) show remarkable chemical and thermal stabilities. For example, h-BN is stable to decomposition at temperatures up to 1000 °C in air, 1400 °C in vacuum, and 2800 °C in an inert atmosphere. The reactivity of h-BN and c-BN is relatively similar, and the data for c-BN are summarized in the table below. Thermal stability of c-BN can be summarized as follows: * In air or oxygen: protective layer prevents further oxidation to ~1300 °C; no conversion to hexagonal form at 1400 °C. * In nitrogen: some conversion to h-BN at 1525 °C after 12 h. * In vacuum (): conversion to h-BN at 1550–1600 °C.Chemical stability
Boron nitride is insoluble in the usual acids, but is soluble in alkaline molten salts and nitrides, such as LiOH, KOH, NaOH- , , , , , or , which are therefore used to etch BN.Thermal conductivity
The theoretical thermal conductivity of hexagonal boron nitride nanoribbons (BNNRs) can approach 1700–2000 W/( m⋅ K), which has the same order of magnitude as the experimental measured value forgraphene
Graphene () is an allotrope of carbon consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice nanostructure.
, and can be comparable to the theoretical calculations for graphene nanoribbons. Moreover, the thermal transport in the BNNRs is anisotropic
Anisotropy () is the property of a material which allows it to change or assume different properties in different directions, as opposed to isotropy. It can be defined as a difference, when measured along different axes, in a material's physic ...
. The thermal conductivity of zigzag-edged BNNRs is about 20% larger than that of armchair-edged nanoribbons at room temperature.
Natural occurrence
In 2009, a naturally occurring boron nitride mineral in the cubic form (c-BN) was reported inTibet
Tibet (; ''Böd''; ) is a region in East Asia, covering much of the Tibetan Plateau and spanning about . It is the traditional homeland of the Tibetan people. Also resident on the plateau are some other ethnic groups such as Monpa people, ...
, and the name qingsongite proposed. The substance was found in dispersed micron
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American spelling), also commonly known as a micron, is a unit of length in the International System of Un ...
-sized inclusions in chromium-rich rocks. In 2013, the International Mineralogical Association affirmed the mineral and the name.
Synthesis
Preparation and reactivity of hexagonal BN
Boron nitride is produced synthetically. Hexagonal boron nitride is obtained by the reacting boron trioxide () or boric acid () withammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous ...
() or urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
() in a nitrogen atmosphere:
: (''T'' = 900 °C)
: (''T'' = 900 °C)
: (''T'' > 1000 °C)
: (''T'' > 1500 °C)
The resulting disordered (amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid, glassy solid) is a solid that lacks the long-range order that is characteristic of a crystal.
Etymology
The term comes from the Greek language, Gr ...
) boron nitride contains 92–95% BN and 5–8% . The remaining can be evaporated in a second step at temperatures in order to achieve BN concentration >98%. Such annealing also crystallizes BN, the size of the crystallites increasing with the annealing temperature.
h-BN parts can be fabricated inexpensively by hot-pressing with subsequent machining. The parts are made from boron nitride powders adding boron oxide for better compressibility. Thin films of boron nitride can be obtained by chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (subst ...
from boron trichloride and nitrogen precursors. Combustion of boron powder in nitrogen plasma at 5500 °C yields ultrafine boron nitride used for lubricants and toner
Toner is a powder mixture used in laser printers and photocopiers to form the printed text and images on paper, in general through a toner cartridge. Mostly granulated plastic, early mixtures only added carbon powder and iron oxide, however, ...
s.
Boron nitride reacts with iodine fluoride in trichlorofluoromethane at −30 °C to produce an extremely sensitive contact explosive, , in low yield.
Boron nitride reacts with nitrides of lithium, alkaline earth metals and lanthanides to form nitridoborate compounds. For example:
:
Intercalation of hexagonal BN
Similar to graphite, various molecules, such as or alkali metals, can be intercalated into hexagonal boron nitride, that is inserted between its layers. Both experiment and theory suggest the intercalation is much more difficult for BN than for graphite.Preparation of cubic BN
Synthesis of c-BN uses same methods as that of diamond: cubic boron nitride is produced by treating hexagonal boron nitride at high pressure and temperature, much assynthetic diamond
Lab-grown diamond (LGD; also called laboratory-grown, laboratory-created, man-made, artisan-created, artificial, synthetic, or cultured diamond) is diamond that is produced in a controlled technological process (in contrast to naturally formed ...
is produced from graphite. Direct conversion of hexagonal boron nitride to the cubic form has been observed at pressures between 5 and 18 GPa and temperatures between 1730 and 3230 °C, that is similar parameters as for direct graphite-diamond conversion. The addition of a small amount of boron oxide can lower the required pressure to 4–7 GPa and temperature to 1500 °C. As in diamond synthesis, to further reduce the conversion pressures and temperatures, a catalyst is added, such as lithium, potassium, or magnesium, their nitrides, their fluoronitrides, water with ammonium compounds, or hydrazine. Other industrial synthesis methods, again borrowed from diamond growth, use crystal growth in a temperature gradient, or explosive shock wave
In physics, a shock wave (also spelled shockwave), or shock, is a type of propagating disturbance that moves faster than the local speed of sound in the medium. Like an ordinary wave, a shock wave carries energy and can propagate through a me ...
. The shock wave method is used to produce material called heterodiamond Heterodiamond is a superhard material containing boron, carbon, and nitrogen (BCN). It is formed at high temperatures and high pressures, e.g., by application of an explosive shock wave to a mixture of diamond and cubic boron nitride (c-BN). The he ...
, a superhard compound of boron, carbon, and nitrogen.
Low-pressure deposition of thin films of cubic boron nitride is possible. As in diamond growth, the major problem is to suppress the growth of hexagonal phases (h-BN or graphite, respectively). Whereas in diamond growth this is achieved by adding hydrogen gas, boron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bond ...
is used for c-BN. Ion beam deposition
Ion beam deposition (IBD) is a process of applying materials to a target through the application of an ion beam.
Ion beam deposition setup with mass separator
An ion beam deposition apparatus typically consists of an ion source, ion optics, and ...
, plasma-enhanced chemical vapor deposition
Plasma-enhanced chemical vapor deposition (PECVD) is a chemical vapor deposition process used to deposit thin films from a gas state (vapor) to a solid state on a substrate. Chemical reactions are involved in the process, which occur after creati ...
, pulsed laser deposition
Pulsed laser deposition (PLD) is a physical vapor deposition (PVD) technique where a high-power pulsed laser beam is focused inside a vacuum chamber to strike a target of the material that is to be deposited. This material is vaporized from the ...
, reactive sputtering, and other physical vapor deposition methods are used as well.
Preparation of wurtzite BN
Wurtzite BN can be obtained via static high-pressure or dynamic shock methods. The limits of its stability are not well defined. Both c-BN and w-BN are formed by compressing h-BN, but formation of w-BN occurs at much lower temperatures close to 1700 °C.Production statistics
Whereas the production and consumption figures for the raw materials used for BN synthesis, namely boric acid and boron trioxide, are well known (seeboron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the '' boron group'' it has t ...
), the corresponding numbers for the boron nitride are not listed in statistical reports. An estimate for the 1999 world production is 300 to 350 metric tons
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
. The major producers and consumers of BN are located in the United States, Japan, China and Germany. In 2000, prices varied from about $75–120/kg for standard industrial-quality h-BN and were about up to $200–400/kg for high purity BN grades.
Applications
Hexagonal BN
Hexagonal BN (h-BN) is the most widely used polymorph. It is a good lubricant at both low and high temperatures (up to 900 °C, even in an oxidizing atmosphere). h-BN lubricant is particularly useful when the electrical conductivity or chemical reactivity of graphite (alternative lubricant) would be problematic. In internal combustion engines, where graphite could be oxidized and turn into carbon sludge, h-BN with its superior thermal stability can be added to engine lubricant, however, with all nano-particles suspension, Brownian-motion settlement is a key problem and settlement can clog engine oil filters, which limits solid lubricants application in a combustion engine to only automotive race settings, where engine re-building is a common practice. Since carbon has appreciable solubility in certain alloys (such as steels), which may lead to degradation of properties, BN is often superior for high temperature and/or high pressure applications. Another advantage of h-BN over graphite is that its lubricity does not require water or gas molecules trapped between the layers. Therefore, h-BN lubricants can be used even in vacuum, e.g. in space applications. The lubricating properties of fine-grained h-BN are used incosmetics
Cosmetics are constituted mixtures of chemical compounds derived from either natural sources, or synthetically created ones. Cosmetics have various purposes. Those designed for personal care and skin care can be used to cleanse or protec ...
, paint
Paint is any pigmented liquid, liquefiable, or solid mastic composition that, after application to a substrate in a thin layer, converts to a solid film. It is most commonly used to protect, color, or provide texture. Paint can be made in many ...
s, dental cements, and pencil
A pencil () is a writing or drawing implement with a solid pigment core in a protective casing that reduces the risk of core breakage, and keeps it from marking the user's hand.
Pencils create marks by physical abrasion, leaving a tra ...
leads.
Hexagonal BN was first used in cosmetics around 1940 in Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the n ...
. However, because of its high price, h-BN was soon abandoned for this application. Its use was revitalized in the late 1990s with the optimization h-BN production processes, and currently h-BN is used by nearly all leading producers of cosmetic products for foundations, make-up
Cosmetics are constituted mixtures of chemical compounds derived from either natural sources, or synthetically created ones. Cosmetics have various purposes. Those designed for personal care and skin care can be used to cleanse or protect ...
, eye shadow
Eye shadow (or eyeshadow) is a cosmetic applied primarily to the eyelids to attract attention to the wearer's eyes, making them stand out or look more attractive. Eye shadow can also be applied under the eyes, on the cheeks, or to brow bones.
...
s, blushers, kohl pencils, lipstick
Lipstick is a cosmetic product used to apply coloration and texture to lips, often made of wax and oil. Different pigments are used to produce color, and minerals such as silica may be used to provide texture. The use of lipstick dates bac ...
s and other skincare products.
Because of its excellent thermal and chemical stability, boron nitride ceramics are traditionally used as parts of high-temperature equipment. h-BN can be included in ceramics, alloys, resins, plastics, rubbers, and other materials, giving them self-lubricating properties. Such materials are suitable for construction of e.g. bearings and in steelmaking. Plastics filled with BN have less thermal expansion as well as higher thermal conductivity and electrical resistivity. Due to its excellent dielectric and thermal properties, BN is used in electronics e.g. as a substrate for semiconductors, microwave-transparent windows, as a heat conductive yet electrically insulating filler in thermal pastes, and as a structural material for seals. Many quantum devices use multilayer h-BN as a substrate material. It can also be used as a dielectric in resistive random access memories.
Hexagonal BN is used in xerographic process and laser printer
Laser printing is an electrostatic digital printing process. It produces high-quality text and graphics (and moderate-quality photographs) by repeatedly passing a laser beam back and forth over a negatively-charged cylinder called a "drum" to ...
s as a charge leakage barrier layer of the photo drum. In the automotive industry, h-BN mixed with a binder (boron oxide) is used for sealing oxygen sensor
An oxygen sensor (or lambda sensor, where lambda refers to air–fuel ratio#Air–fuel equivalence ratio (λ), air–fuel equivalence ratio, usually denoted by λ) or probe or wikt:sond, sond, is an electronics, electronic device that measures th ...
s, which provide feedback for adjusting fuel flow. The binder utilizes the unique temperature stability and insulating properties of h-BN.
Parts can be made by hot pressing from four commercial grades of h-BN. Grade HBN contains a boron oxide binder; it is usable up to 550–850 °C in oxidizing atmosphere and up to 1600 °C in vacuum, but due to the boron oxide content is sensitive to water. Grade HBR uses a calcium borate
Calcium borate (Ca3(BO3)2), also called Gerstley borate, is a bluish white crystal with a very defined structure. It can be prepared by reacting calcium metal with boric acid. The resulting precipitate is calcium borate. A hydrated form occurs na ...
binder and is usable at 1600 °C. Grades HBC and HBT contain no binder and can be used up to 3000 °C.
Boron nitride nanosheets (h-BN) can be deposited by catalytic decomposition of borazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For th ...
at a temperature ~1100 °C in a chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (subst ...
setup, over areas up to about 10 cm2. Owing to their hexagonal atomic structure, small lattice mismatch with graphene (~2%), and high uniformity they are used as substrates for graphene-based devices. BN nanosheets are also excellent proton conductor
A proton conductor is an electrolyte, typically a solid electrolyte, in which H+ are the primary charge carriers.
Composition
Acid solutions exhibit proton-conductivity, while pure proton conductors are usually dry solids. Typical materials a ...
s. Their high proton transport rate, combined with the high electrical resistance, may lead to applications in fuel cells
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
and water electrolysis.
h-BN has been used since the mid-2000s as a bullet and bore lubricant in precision target rifle applications as an alternative to molybdenum disulfide
Molybdenum disulfide (or moly) is an inorganic compound composed of molybdenum and sulfur. Its chemical formula is .
The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as the mineral molybdeni ...
coating, commonly referred to as "moly". It is claimed to increase effective barrel life, increase intervals between bore cleaning, and decrease the deviation in point of impact between clean bore first shots and subsequent shots.
Cubic BN
Cubic boron nitride (CBN or c-BN) is widely used as an abrasive. Its usefulness arises from its insolubility iniron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, nickel
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow t ...
, and related alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductili ...
s at high temperatures, whereas diamond is soluble in these metals. Polycrystalline c-BN (PCBN) abrasives are therefore used for machining steel, whereas diamond abrasives are preferred for aluminum alloys, ceramics, and stone. When in contact with oxygen at high temperatures, BN forms a passivation layer of boron oxide. Boron nitride binds well with metals, due to formation of interlayers of metal borides or nitrides. Materials with cubic boron nitride crystals are often used in the tool bit
A tool bit is a non-rotary cutting tool used in metal lathes, shapers, and planers. Such cutters are also often referred to by the set-phrase name of single-point cutting tool, as distinguished from other cutting tools such as a saw or water j ...
s of cutting tools. For grinding applications, softer binders, e.g. resin, porous ceramics, and soft metals, are used. Ceramic binders can be used as well. Commercial products are known under names "Borazon
Borazon is a brand name of a cubic form of boron nitride (cBN). Its color ranges from black to brown and gold, depending on the chemical bond. It is one of the hardest known materials, along with various forms of diamond and kinds of boron nitride ...
" (by Hyperion Materials & Technologies), and "Elbor" or "Cubonite" (by Russian vendors).
Contrary to diamond, large c-BN pellets can be produced in a simple process (called sintering) of annealing c-BN powders in nitrogen flow at temperatures slightly below the BN decomposition temperature. This ability of c-BN and h-BN powders to fuse allows cheap production of large BN parts.
Similar to diamond, the combination in c-BN of highest thermal conductivity and electrical resistivity is ideal for heat spreaders.
As cubic boron nitride consists of light atoms and is very robust chemically and mechanically, it is one of the popular materials for X-ray membranes: low mass results in small X-ray absorption, and good mechanical properties allow usage of thin membranes, thus further reducing the absorption.
Amorphous BN
Layers of amorphous boron nitride (a-BN) are used in somesemiconductor device
A semiconductor device is an electronic component that relies on the electronic properties of a semiconductor material (primarily silicon, germanium, and gallium arsenide, as well as organic semiconductors) for its function. Its conductivit ...
s, e.g. MOSFET
The metal–oxide–semiconductor field-effect transistor (MOSFET, MOS-FET, or MOS FET) is a type of field-effect transistor (FET), most commonly fabricated by the controlled oxidation of silicon. It has an insulated gate, the voltage of which d ...
s. They can be prepared by chemical decomposition of trichloroborazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For th ...
with caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
, or by thermal chemical vapor deposition methods. Thermal CVD can be also used for deposition of h-BN layers, or at high temperatures, c-BN.
Other forms of boron nitride
Atomically thin boron nitride
Hexagonal boron nitride can be exfoliated to mono or few atomic layer sheets. Due to its analogous structure to that of graphene, atomically thin boron nitride is sometimes called ''white graphene''.Mechanical properties
Atomically thin boron nitride is one of the strongest electrically insulating materials. Monolayer boron nitride has an average Young's modulus of 0.865TPa and fracture strength of 70.5GPa, and in contrast to graphene, whose strength decreases dramatically with increased thickness, few-layer boron nitride sheets have a strength similar to that of monolayer boron nitride.Thermal conductivity
Atomically thin boron nitride has one of the highest thermal conductivity coefficients (751 W/mK at room temperature) among semiconductors and electrical insulators, and its thermal conductivity increases with reduced thickness due to less intra-layer coupling.Thermal stability
The air stability of graphene shows a clear thickness dependence: monolayer graphene is reactive to oxygen at 250 °C, strongly doped at 300 °C, and etched at 450 °C; in contrast, bulk graphite is not oxidized until 800 °C. Atomically thin boron nitride has much better oxidation resistance than graphene. Monolayer boron nitride is not oxidized till 700 °C and can sustain up to 850 °C in air; bilayer and trilayer boron nitride nanosheets have slightly higher oxidation starting temperatures. The excellent thermal stability, high impermeability to gas and liquid, and electrical insulation make atomically thin boron nitride potential coating materials for preventing surface oxidation and corrosion of metals and other two-dimensional (2D) materials, such asblack phosphorus
Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
White ...
.
Better surface adsorption
Atomically thin boron nitride has been found to have better surface adsorption capabilities than bulk hexagonal boron nitride. According to theoretical and experimental studies, atomically thin boron nitride as an adsorbent experiences conformational changes upon surface adsorption of molecules, increasing adsorption energy and efficiency. The synergic effect of the atomic thickness, high flexibility, stronger surface adsorption capability, electrical insulation, impermeability, high thermal and chemical stability of BN nanosheets can increase the Raman sensitivity by up to two orders, and in the meantime attain long-term stability and extraordinary reusability not achievable by other materials.Dielectric properties
Atomically thin hexagonal boron nitride is an excellent dielectric substrate for graphene, molybdenum disulfide (), and many other 2D material-based electronic and photonic devices. As shown by electric force microscopy (EFM) studies, the electric field screening in atomically thin boron nitride shows a weak dependence on thickness, which is in line with the smooth decay of electric field inside few-layer boron nitride revealed by the first-principles calculations.Raman characteristics
Raman spectroscopy has been a useful tool to study a variety of 2D materials, and the Raman signature of high-quality atomically thin boron nitride was first reported by Gorbachev et al. in 2011. and Li et al. However, the two reported Raman results of monolayer boron nitride did not agree with each other. Cai et al., therefore, conducted systematic experimental and theoretical studies to reveal the intrinsic Raman spectrum of atomically thin boron nitride. It reveals that atomically thin boron nitride without interaction with a substrate has a G band frequency similar to that of bulk hexagonal boron nitride, but strain induced by the substrate can cause Raman shifts. Nevertheless, the Raman intensity of G band of atomically thin boron nitride can be used to estimate layer thickness and sample quality.
Boron nitride nanomesh
Boron nitride nanomesh is a nanostructured two-dimensional material. It consists of a single BN layer, which forms byself-assembly
Self-assembly is a process in which a disordered system of pre-existing components forms an organized structure or pattern as a consequence of specific, local interactions among the components themselves, without external direction. When the ...
a highly regular mesh after high-temperature exposure of a clean rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring i ...
or ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
surface to borazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For th ...
under ultra-high vacuum
Ultra-high vacuum (UHV) is the vacuum regime characterised by pressures lower than about . UHV conditions are created by pumping the gas out of a UHV chamber. At these low pressures the mean free path of a gas molecule is greater than approximatel ...
. The nanomesh looks like an assembly of hexagonal pores. The distance between two pore centers is 3.2 nm and the pore diameter is ~2 nm. Other terms for this material are boronitrene or white graphene.
The boron nitride nanomesh is not only stable to decomposition under vacuum, air and some liquids, but also up to temperatures of 800 °C. In addition, it shows the extraordinary ability to trap molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
s and metallic clusters which have similar sizes to the nanomesh pores, forming a well-ordered array. These characteristics promise interesting applications of the nanomesh in areas like catalysis, surface functionalisation, spintronics
Spintronics (a portmanteau meaning spin transport electronics), also known as spin electronics, is the study of the intrinsic spin of the electron and its associated magnetic moment, in addition to its fundamental electronic charge, in solid- ...
, quantum computing
Quantum computing is a type of computation whose operations can harness the phenomena of quantum mechanics, such as superposition, interference, and entanglement. Devices that perform quantum computations are known as quantum computers. Though ...
and data storage media like hard drive
A hard disk drive (HDD), hard disk, hard drive, or fixed disk is an electro-mechanical data storage device that stores and retrieves digital data using magnetic storage with one or more rigid rapidly rotating platters coated with mag ...
s.
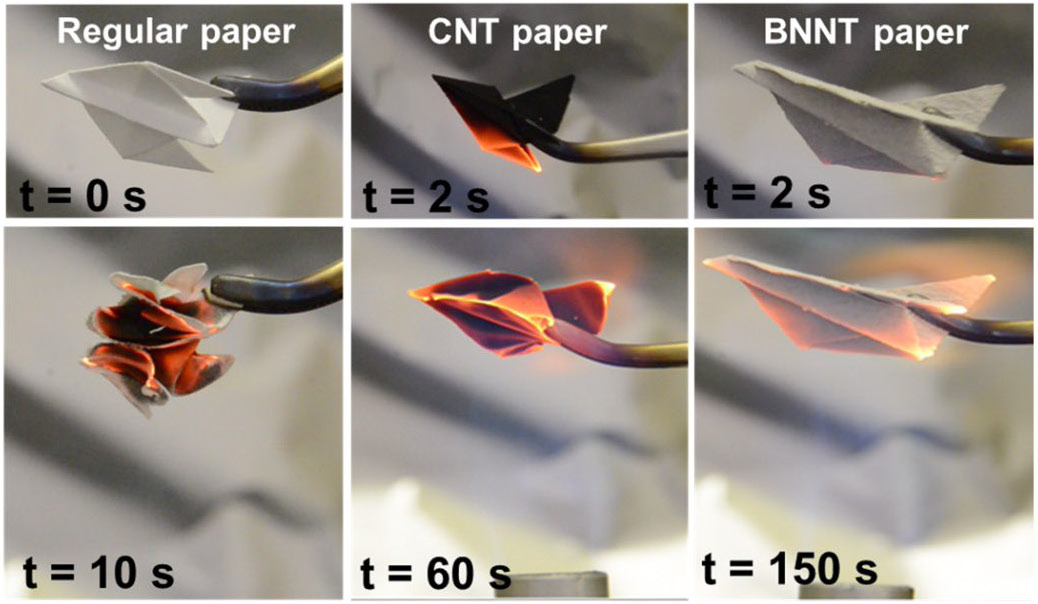
Boron nitride nanotubes
Boron nitride tubules were first made in 1989 by Shore and Dolan This work was patented in 1989 and published in 1989 thesis (Dolan) and then 1993 Science. The 1989 work was also the first preparation of amorphous BN by B-trichloroborazine and cesium metal. Boron nitride nanotubes were predicted in 1994 and experimentally discovered in 1995. They can be imagined as a rolled up sheet of h-boron nitride. Structurally, it is a close analog of thecarbon nanotube
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon na ...
, namely a long cylinder with diameter of several to hundred nanometers and length of many micrometers, except carbon atoms are alternately substituted by nitrogen and boron atoms. However, the properties of BN nanotubes are very different: whereas carbon nanotubes can be metallic or semiconducting depending on the rolling direction and radius, a BN nanotube is an electrical insulator with a bandgap of ~5.5 eV, basically independent of tube chirality and morphology. In addition, a layered BN structure is much more thermally and chemically stable than a graphitic carbon structure.
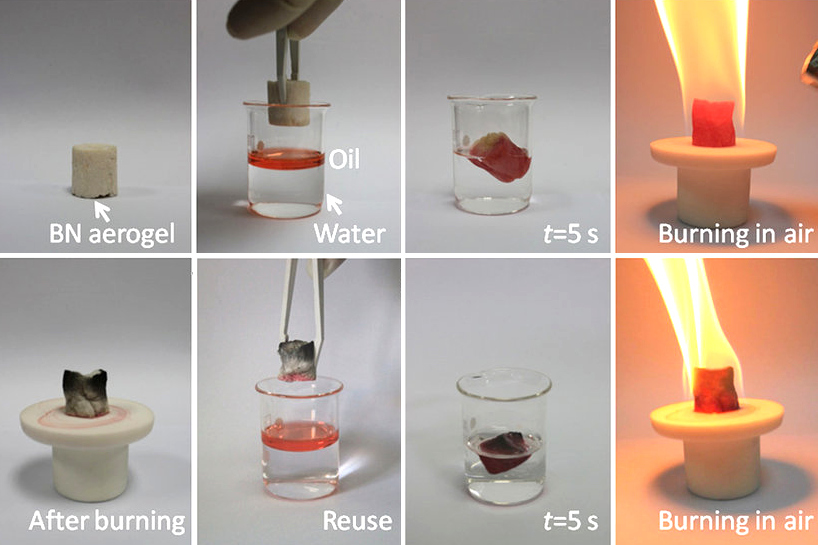
Boron nitride aerogel
Boron nitride aerogel is anaerogel
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low ...
made of highly porous BN. It typically consists of a mixture of deformed BN nanotubes and nanosheets. It can have a density as low as 0.6 mg/cm3 and a specific surface area as high as 1050 m2/g, and therefore has potential applications as an absorbent, catalyst support and gas storage medium. BN aerogels are highly hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, ...
and can absorb up to 160 times their weight in oil. They are resistant to oxidation in air at temperatures up to 1200 °C, and hence can be reused after the absorbed oil is burned out by flame. BN aerogels can be prepared by template-assisted chemical vapor deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films.
In typical CVD, the wafer (subst ...
using borazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For th ...
as the feed gas.
Composites containing BN
Addition of boron nitride tosilicon nitride
Silicon nitride is a chemical compound of the elements silicon and nitrogen. is the most thermodynamically stable and commercially important of the silicon nitrides, and the term "silicon nitride" commonly refers to this specific composition. It ...
ceramics improves the thermal shock resistance of the resulting material. For the same purpose, BN is added also to silicon nitride- alumina and titanium nitride
Titanium nitride (TiN; sometimes known as Tinite) is an extremely hard ceramic material, often used as a physical vapor deposition (PVD) coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface prop ...
-alumina ceramics. Other materials being reinforced with BN include alumina and zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant ...
, borosilicate glasses, glass ceramic
Glass-ceramics are polycrystalline materials produced through controlled crystallization of base glass, producing a fine uniform dispersion of crystals throughout the bulk material. Crystallization is accomplished by subjecting suitable glasses to ...
s, enamels, and composite ceramics with titanium boride-boron nitride, titanium boride-aluminium nitride
Aluminium nitride ( Al N) is a solid nitride of aluminium. It has a high thermal conductivity of up to 321 W/(m·K) and is an electrical insulator. Its wurtzite phase (w-AlN) has a band gap of ~6 eV at room temperature and has a potenti ...
-boron nitride, and silicon carbide
Silicon carbide (SiC), also known as carborundum (), is a hard chemical compound containing silicon and carbon. A semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal s ...
-boron nitride composition.
Health issues
Boron nitride (along with , NbN, and BNC) is reported to show weakfibrogenic
Fibrosis, also known as fibrotic scarring, is a pathological wound healing in which connective tissue replaces normal parenchymal tissue to the extent that it goes unchecked, leading to considerable tissue remodelling and the formation of perman ...
activity, and to cause pneumoconiosis
Pneumoconiosis is the general term for a class of interstitial lung disease where inhalation of dust ( for example, ash dust, lead particles, pollen grains etc) has caused interstitial fibrosis. The three most common types are asbestosis, silico ...
when inhaled in particulate form. The maximum concentration recommended for nitrides of nonmetals is 10 mg/m3 for BN and 4 for AlN or ZrN.
See also
* Beta carbon nitride * Borocarbonitrides * Boron suboxide *Superhard materials
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their ...
* Wide-bandgap semiconductor
Wide-bandgap semiconductors (also known as WBG semiconductors or WBGSs) are semiconductor materials which have a larger band gap than conventional semiconductors. Conventional semiconductors like silicon have a bandgap in the range of 0.6 � ...
s
Notes
References
External links
National Pollutant Inventory: Boron and Compounds
at University of Oxford {{DEFAULTSORT:Boron Nitride Boron compounds Ceramic materials Nitrides III-V semiconductors Non-petroleum based lubricants Dry lubricants Abrasives Superhard materials Neutron poisons Monolayers III-V compounds Boron–nitrogen compounds Zincblende crystal structure Wurtzite structure type