Biogeochemical cycle on:
[Wikipedia]
[Google]
[Amazon]
A biogeochemical cycle (or more generally a cycle of matter) is the pathway by which a
 Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.Biogeochemical Cycles
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.Biogeochemical Cycles
, ''OpenStax'', 9 May 2019. Material was copied from this source, which is available under
Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of nucleic acids and proteins. Phosphorus is used to make nucleic acids and the phospholipids that comprise biological membranes. Sulfur is critical to the three-dimensional shape of proteins. The cycling of these elements is interconnected. For example, the movement of water is critical for leaching sulfur and phosphorus into rivers which can then flow into oceans. Minerals cycle through the biosphere between the biotic and abiotic components and from one organism to another.Fisher M. R. (Ed.) (2019) ''Environmental Biology''
3.2 Biogeochemical Cycles
, OpenStax. Material was copied from this source, which is available under
Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. Ecological systems (
Biogeochemical cycles
. ''
File:BIOGEOCHEMICAL CYCLING OF ELEMENTS.svg,
File:WhalePump.jpg, The oceanic whale pump showing how whales cycle nutrients through the ocean
Although the Earth constantly receives energy from the sun, its chemical composition is essentially fixed, as the additional matter is only occasionally added by meteorites. Because this chemical composition is not replenished like energy, all processes that depend on these chemicals must be recycled. These cycles include both the living biosphere and the nonliving lithosphere, atmosphere, and hydrosphere.
Biogeochemical cycles can be contrasted with

 The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and
The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and  Material was copied from this source, which is available under
Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. Global change is, therefore, affecting key processes including
 Material was copied from this source, which is available under
Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
.
 Box models are widely used to model biogeochemical systems. Bianchi, Thomas (2007
Box models are widely used to model biogeochemical systems. Bianchi, Thomas (2007
''Biogeochemistry of Estuaries''
page 9, Oxford University Press. . Box models are simplified versions of complex systems, reducing them to boxes (or storage
 The diagram on the left above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the
The diagram on the left above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the  Material was copied from this source, which is available under
Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
.
Blue planet: The role of the oceans in nutrient cycling, maintain the atmosphere system, and modulating climate change
In: ''Routledge Handbook of Ocean Resources and Management'', Routledge, pages 89–107. . As an example, the fast carbon cycle is illustrated in the diagram below on the left. This cycle involves relatively short-term Material was copied from this source, which is available under
Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
. The slow cycle is illustrated in the diagram above on the right. It involves medium to long-term geochemical processes belonging to the
The slow cycle is illustrated in the diagram above on the right. It involves medium to long-term geochemical processes belonging to the
 Material was copied from this source, which is available under
Material was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
.
File:Carbon cycle-cute diagram.svg, alt=Diagram of the carbon cycle,
Many biogeochemical cycles are currently being studied for the first time.
File:Plagiomnium affine laminazellen.jpeg,
Biogeochemical cycles always involve active equilibrium states: a balance in the cycling of the element between compartments. However, overall balance may involve compartments distributed on a global scale.
As biogeochemical cycles describe the movements of substances on the entire globe, the study of these is inherently multidisciplinary. The carbon cycle may be related to research in
DOI 10.1515/9783110589771-002
* * * * {{DEFAULTSORT:Biogeochemical Cycle Geochemistry Biogeography
chemical substance
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., w ...
cycles (is turned over or moves through) the biotic and the abiotic
In biology and ecology, abiotic components or abiotic factors are non-living chemical and physical parts of the environment that affect living organisms and the functioning of ecosystems. Abiotic factors and the phenomena associated with them under ...
compartments of Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's surfa ...
. The biotic compartment is the biosphere
The biosphere (from Greek βίος ''bíos'' "life" and σφαῖρα ''sphaira'' "sphere"), also known as the ecosphere (from Greek οἶκος ''oîkos'' "environment" and σφαῖρα), is the worldwide sum of all ecosystems. It can also ...
and the abiotic compartments are the atmosphere, hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to change in shape. This ...
and lithosphere. There are biogeochemical
Biogeochemistry is the scientific discipline that involves the study of the chemical, physical, geological, and biological processes and reactions that govern the composition of the natural environment (including the biosphere, the cryosphere, th ...
cycles for chemical elements, such as for calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar t ...
, carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
, hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, mercury, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
, selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
, iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
and sulfur, as well as molecular cycles, such as for water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is ...
. There are also macroscopic cycles, such as the rock cycle
The rock cycle is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium conditi ...
, and human-induced cycles for synthetic compounds such as polychlorinated biphenyl
Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act in 1979 and internationally by t ...
s (PCBs). In some cycles there are reservoirs where a substance can remain or be sequestered for a long period of time.
Overview
 Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.Biogeochemical Cycles
Energy flows directionally through ecosystems, entering as sunlight (or inorganic molecules for chemoautotrophs) and leaving as heat during the many transfers between trophic levels. However, the matter that makes up living organisms is conserved and recycled. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in the atmosphere, on land, in water, or beneath the Earth's surface. Geologic processes, such as weathering, erosion, water drainage, and the subduction of the continental plates, all play a role in this recycling of materials. Because geology and chemistry have major roles in the study of this process, the recycling of inorganic matter between living organisms and their environment is called a biogeochemical cycle.Biogeochemical Cycles, ''OpenStax'', 9 May 2019.
Creative Commons Attribution 4.0 International License
. The six aforementioned elements are used by organisms in a variety of ways. Hydrogen and oxygen are found in water and organic molecules, both of which are essential to life. Carbon is found in all organic molecules, whereas nitrogen is an important component of nucleic acids and proteins. Phosphorus is used to make nucleic acids and the phospholipids that comprise biological membranes. Sulfur is critical to the three-dimensional shape of proteins. The cycling of these elements is interconnected. For example, the movement of water is critical for leaching sulfur and phosphorus into rivers which can then flow into oceans. Minerals cycle through the biosphere between the biotic and abiotic components and from one organism to another.Fisher M. R. (Ed.) (2019) ''Environmental Biology''
3.2 Biogeochemical Cycles
, OpenStax.
Creative Commons Attribution 4.0 International License
. Ecological systems (
ecosystem
An ecosystem (or ecological system) consists of all the organisms and the physical environment with which they interact. These biotic and abiotic components are linked together through nutrient cycles and energy flows. Energy enters the syste ...
s) have many biogeochemical cycles operating as a part of the system, for example, the water cycle, the carbon cycle, the nitrogen cycle, etc. All chemical elements occurring in organisms are part of biogeochemical cycles. In addition to being a part of living organisms, these chemical elements also cycle through abiotic factors of ecosystems such as water (hydrosphere
The hydrosphere () is the combined mass of water found on, under, and above the surface of a planet, minor planet, or natural satellite. Although Earth's hydrosphere has been around for about 4 billion years, it continues to change in shape. This ...
), land ( lithosphere), and/or the air ( atmosphere).
The living factors of the planet can be referred to collectively as the biosphere. All the nutrients—such as carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
, and sulfur—used in ecosystems by living organisms are a part of a ''closed system''; therefore, these chemicals are recycled instead of being lost and replenished constantly such as in an open system.
The diagram on the right shows a generalised biogeochemical cycle. The major parts of the biosphere are connected by the flow of chemical elements and compounds. In many of these cycles, the biota plays an important role. Matter from the Earth's interior is released by volcanoes. The atmosphere exchanges some compounds and elements rapidly with the biota and oceans. Exchanges of materials between rocks, soils, and the oceans are generally slower by comparison.Moses, M. (2012Biogeochemical cycles
. ''
Encyclopedia of Earth
The ''Encyclopedia of Earth'' (abbreviated ''EoE'') is an electronic reference about the Earth, its natural environments, and their interaction with society. The ''Encyclopedia'' is described as a free, fully searchable collection of articles ...
''.
The flow of energy in an ecosystem is an ''open system''; the sun constantly gives the planet energy in the form of light while it is eventually used and lost in the form of heat throughout the trophic level
The trophic level of an organism is the position it occupies in a food web. A food chain is a succession of organisms that eat other organisms and may, in turn, be eaten themselves. The trophic level of an organism is the number of steps it ...
s of a food web. Carbon is used to make carbohydrates, fats, and proteins, the major sources of food energy
Food energy is chemical energy that animals (including humans) derive from their food to sustain their metabolism, including their muscular activity.
Most animals derive most of their energy from aerobic respiration, namely combining the carbohy ...
. These compounds are oxidized to release carbon dioxide, which can be captured by plants to make organic compounds. The chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
is powered by the light energy of the sun.
Sunlight is required to combine carbon with hydrogen and oxygen into an energy source, but ecosystems in the deep sea
The deep sea is broadly defined as the ocean depth where light begins to fade, at an approximate depth of 200 metres (656 feet) or the point of transition from continental shelves to continental slopes. Conditions within the deep sea are a combin ...
, where no sunlight can penetrate, obtain energy from sulfur. Hydrogen sulfide near hydrothermal vents can be utilized by organisms such as the giant tube worm
''Riftia pachyptila'', commonly known as the giant tube worm and less commonly known as the Giant beardworm, is a marine invertebrate in the phylum Annelida (formerly grouped in phylum Pogonophora and Vestimentifera) related to tube worm ...
. In the sulfur cycle, sulfur can be forever recycled as a source of energy. Energy can be released through the oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
and reduction of sulfur compounds (e.g., oxidizing elemental sulfur to sulfite and then to sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
).
water column
A water column is a conceptual column of water from the surface of a sea, river or lake to the bottom sediment.Munson, B.H., Axler, R., Hagley C., Host G., Merrick G., Richards C. (2004).Glossary. ''Water on the Web''. University of Minnesota-D ...
File:Global carbon cycle.webp, The implications of shifts in the global carbon cycle due to human activity are concerning scientists.
geochemical cycle In Earth science, a geochemical cycle is the pathway that chemical elements take in the surface and crust of the Earth. The term "geochemical" tells us that geological and chemical factors are all included. The migration of heated and compressed che ...
s. The latter deals only with crustal and subcrustal reservoirs even though some process from both overlap.
Compartments
Atmosphere
Hydrosphere

 The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and
The global ocean covers more than 70% of the Earth's surface and is remarkably heterogeneous. Marine productive areas, and coastal ecosystem
A marine coastal ecosystem is a marine ecosystem which occurs where the land meets the ocean. Marine coastal ecosystems include many different types of marine habitats, such as estuaries and lagoons, salt marshes and mangrove forests, seagrass me ...
s comprise a minor fraction of the ocean in terms of surface area, yet have an enormous impact on global biogeochemical cycles carried out by microbial communities Microbial population biology is the application of the principles of population biology to microorganisms.
Distinguishing from other biological disciplines
Microbial population biology, in practice, is the application of population ecology and popu ...
, which represent 90% of the ocean's biomass. Work in recent years has largely focused on cycling of carbon and macronutrients such as nitrogen, phosphorus, and silicate: other important elements such as sulfur or trace elements have been less studied, reflecting associated technical and logistical issues. Increasingly, these marine areas, and the taxa that form their ecosystems, are subject to significant anthropogenic pressure, impacting marine life and recycling of energy and nutrients. A key example is that of cultural eutrophication
Eutrophication is the process by which an entire body of water, or parts of it, becomes progressively enriched with minerals and nutrients, particularly nitrogen and phosphorus. It has also been defined as "nutrient-induced increase in phytoplank ...
, where agricultural runoff
Agricultural pollution refers to biotic and abiotic byproducts of farming practices that result in contamination or degradation of the environment and surrounding ecosystems, and/or cause injury to humans and their economic interests. The pol ...
leads to nitrogen and phosphorus enrichment of coastal ecosystems, greatly increasing productivity resulting in algal bloom
An algal bloom or algae bloom is a rapid increase or accumulation in the population of algae in freshwater or marine water systems. It is often recognized by the discoloration in the water from the algae's pigments. The term ''algae'' encompass ...
s, deoxygenation
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to orga ...
of the water column and seabed, and increased greenhouse gas emissions, with direct local and global impacts on nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and carbon cycle
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major componen ...
s. However, the runoff of organic matter from the mainland to coastal ecosystem
A marine coastal ecosystem is a marine ecosystem which occurs where the land meets the ocean. Marine coastal ecosystems include many different types of marine habitats, such as estuaries and lagoons, salt marshes and mangrove forests, seagrass me ...
s is just one of a series of pressing threats stressing microbial communities due to global change. Climate change has also resulted in changes in the cryosphere
]
The cryosphere (from the Ancient Greek, Greek ''kryos'', "cold", "frost" or "ice" and ''sphaira'', "globe, ball") is an all-encompassing term for those portions of Earth's surface where water is in solid form, including sea ice, lake ice, ri ...
, as glaciers and permafrost melt, resulting in intensified Stratification (water), marine stratification, while shifts of the redox-state in different biomes are rapidly reshaping microbial assemblages at an unprecedented rate. Creative Commons Attribution 4.0 International License
. Global change is, therefore, affecting key processes including
primary productivity
In ecology, primary production is the synthesis of organic compounds from atmospheric or aqueous carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through c ...
, CO2 and N2 fixation, organic matter respiration/remineralization
In biogeochemistry, remineralisation (or remineralization) refers to the breakdown or transformation of organic matter (those molecules derived from a biological source) into its simplest inorganic forms. These transformations form a crucial link ...
, and the sinking and burial deposition of fixed CO2. In addition to this, oceans are experiencing an acidification process, with a change of ~0.1 pH units between the pre-industrial period and today, affecting carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
/bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochem ...
buffer
Buffer may refer to:
Science
* Buffer gas, an inert or nonflammable gas
* Buffer solution, a solution used to prevent changes in pH
* Buffering agent, the weak acid or base in a buffer solution
* Lysis buffer, in cell biology
* Metal ion buffer
* ...
chemistry. In turn, acidification has been reported to impact planktonic communities, principally through effects on calcifying taxa. There is also evidence for shifts in the production of key intermediary volatile products, some of which have marked greenhouse effects (e.g., N2O and CH4, reviewed by Breitburg in 2018, due to the increase in global temperature, ocean stratification and deoxygenation, driving as much as 25 to 50% of nitrogen loss from the ocean to the atmosphere in the so-called oxygen minimum zone
The oxygen minimum zone (OMZ), sometimes referred to as the shadow zone, is the zone in which oxygen saturation in seawater in the ocean is at its lowest. This zone occurs at depths of about , depending on local circumstances. OMZs are found worl ...
s or anoxic
The term anoxia means a total depletion in the level of oxygen, an extreme form of hypoxia or "low oxygen". The terms anoxia and hypoxia are used in various contexts:
* Anoxic waters, sea water, fresh water or groundwater that are depleted of diss ...
marine zones, driven by microbial processes. Other products, that are typically toxic for the marine nekton
Nekton or necton (from the ) refers to the actively swimming aquatic organisms in a body of water. The term was proposed by German biologist Ernst Haeckel to differentiate between the active swimmers in a body of water, and the passive organisms t ...
, including reduced sulfur species such as H2S, have a negative impact for marine resources like fisheries and coastal aquaculture. While global change has accelerated, there has been a parallel increase in awareness of the complexity of marine ecosystems, and especially the fundamental role of microbes as drivers of ecosystem functioning.
Lithosphere
Biosphere
Microorganisms drive much of the biogeochemical cycling in the earth system.Creative Commons Attribution 4.0 International License
.
Reservoirs
The chemicals are sometimes held for long periods of time in one place. This place is called a ''reservoir'', which, for example, includes such things ascoal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal is formed when ...
deposits that are storing carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon mak ...
for a long period of time. When chemicals are held for only short periods of time, they are being held in ''exchange pools''. Examples of exchange pools include plants and animals.
Plants and animals utilize carbon to produce carbohydrates, fats, and proteins, which can then be used to build their internal structures or to obtain energy. Plants and animals temporarily use carbon in their systems and then release it back into the air or surrounding medium. Generally, reservoirs are abiotic factors whereas exchange pools are biotic factors. Carbon is held for a relatively short time in plants and animals in comparison to coal deposits. The amount of time that a chemical is held in one place is called its residence time
The residence time of a fluid parcel is the total time that the parcel has spent inside a control volume (e.g.: a chemical reactor, a lake, a human body). The residence time of a set of parcels is quantified in terms of the frequency distributi ...
or turnover time (also called the renewal time or exit age).
Box models
 Box models are widely used to model biogeochemical systems. Bianchi, Thomas (2007
Box models are widely used to model biogeochemical systems. Bianchi, Thomas (2007''Biogeochemistry of Estuaries''
page 9, Oxford University Press. . Box models are simplified versions of complex systems, reducing them to boxes (or storage
reservoir
A reservoir (; from French ''réservoir'' ) is an enlarged lake behind a dam. Such a dam may be either artificial, built to store fresh water or it may be a natural formation.
Reservoirs can be created in a number of ways, including contro ...
s) for chemical materials, linked by material fluxes (flows). Simple box models have a small number of boxes with properties, such as volume, that do not change with time. The boxes are assumed to behave as if they were mixed homogeneously. These models are often used to derive analytical formulas describing the dynamics and steady-state abundance of the chemical species involved.
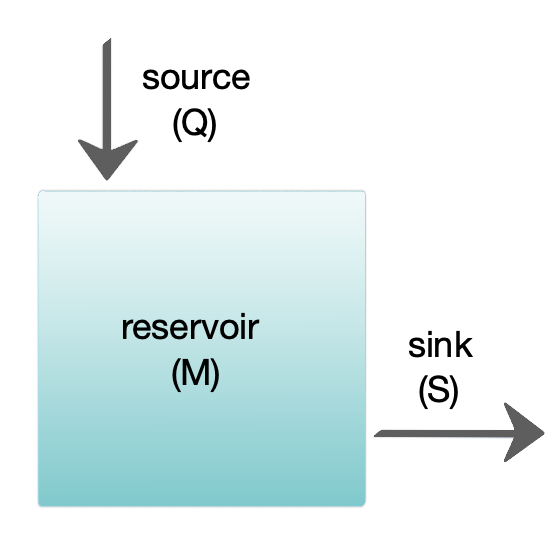
The diagram at the right shows a basic one-box model. The reservoir contains the amount of material ''M'' under consideration, as defined by chemical, physical or biological properties. The source ''Q'' is the flux of material into the reservoir, and the sink ''S'' is the flux of material out of the reservoir. The budget is the check and balance of the sources and sinks affecting material turnover in a reservoir. The reservoir is in a steady state
In systems theory, a system or a process is in a steady state if the variables (called state variables) which define the behavior of the system or the process are unchanging in time. In continuous time, this means that for those properties ''p' ...
if ''Q'' = ''S'', that is, if the sources balance the sinks and there is no change over time.
The residence or turnover time is the average time material spends resident in the reservoir. If the reservoir is in a steady state, this is the same as the time it takes to fill or drain the reservoir. Thus, if τ is the turnover time, then τ = M/S. The equation describing the rate of change of content in a reservoir is
::
When two or more reservoirs are connected, the material can be regarded as cycling between the reservoirs, and there can be predictable patterns to the cyclic flow. More complex multibox models are usually solved using numerical techniques.

 The diagram on the left above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the
The diagram on the left above shows a simplified budget of ocean carbon flows. It is composed of three simple interconnected box models, one for the euphotic zone
The photic zone, euphotic zone, epipelagic zone, or sunlight zone is the uppermost layer of a body of water that receives sunlight, allowing phytoplankton to perform photosynthesis. It undergoes a series of physical, chemical, and biological proc ...
, one for the ocean interior or dark ocean, and one for ocean sediment
Marine sediment, or ocean sediment, or seafloor sediment, are deposits of insoluble particles that have accumulated on the seafloor. These particles have their origins in soil and rocks and have been transported from the land to the sea, mai ...
s. In the euphotic zone, net phytoplankton production is about 50 Pg C each year. About 10 Pg is exported to the ocean interior while the other 40 Pg is respired. Organic carbon degradation occurs as particles
In the physical sciences, a particle (or corpuscule in older texts) is a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass.
They vary greatly in size or quantity, from s ...
(marine snow
In the deep ocean, marine snow (also known as "ocean dandruff") is a continuous shower of mostly organic detritus falling from the upper layers of the water column. It is a significant means of exporting energy from the light-rich photic zone to ...
) settle through the ocean interior. Only 2 Pg eventually arrives at the seafloor, while the other 8 Pg is respired in the dark ocean. In sediments, the time scale available for degradation increases by orders of magnitude with the result that 90% of the organic carbon delivered is degraded and only 0.2 Pg C yr−1 is eventually buried and transferred from the biosphere to the geosphere.
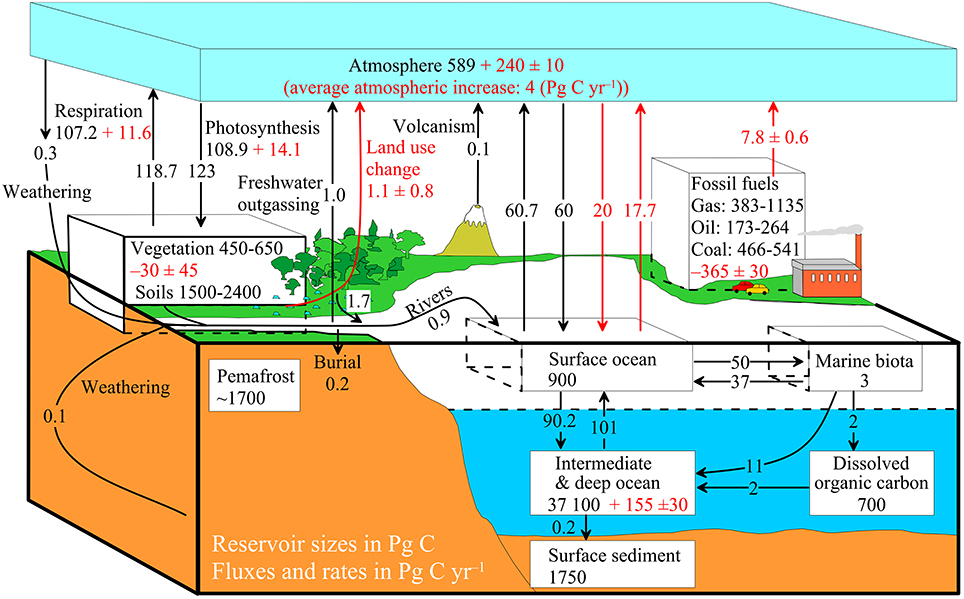
The diagram on the right above shows a more complex model with many interacting boxes. Reservoir masses here represents ''carbon stocks'', measured in Pg C. Carbon exchange fluxes, measured in Pg C yr−1, occur between the atmosphere and its two major sinks, the land and the ocean. The black numbers and arrows indicate the reservoir mass and exchange fluxes estimated for the year 1750, just before the Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going f ...
. The red arrows (and associated numbers) indicate the annual flux changes due to anthropogenic activities, averaged over the 2000–2009 time period. They represent how the carbon cycle has changed since 1750. Red numbers in the reservoirs represent the cumulative changes in anthropogenic carbon since the start of the Industrial Period, 1750–2011. Creative Commons Attribution 4.0 International License
.
Fast and slow cycles
There are fast and slow biogeochemical cycles. Fast cycle operate in thebiosphere
The biosphere (from Greek βίος ''bíos'' "life" and σφαῖρα ''sphaira'' "sphere"), also known as the ecosphere (from Greek οἶκος ''oîkos'' "environment" and σφαῖρα), is the worldwide sum of all ecosystems. It can also ...
and slow cycles operate in rocks. Fast or biological cycles can complete within years, moving substances from atmosphere to biosphere, then back to the atmosphere. Slow or geological cycles can take millions of years to complete, moving substances through the Earth's crust between rocks, soil, ocean and atmosphere.Libes, Susan M. (2015)Blue planet: The role of the oceans in nutrient cycling, maintain the atmosphere system, and modulating climate change
In: ''Routledge Handbook of Ocean Resources and Management'', Routledge, pages 89–107. . As an example, the fast carbon cycle is illustrated in the diagram below on the left. This cycle involves relatively short-term
biogeochemical
Biogeochemistry is the scientific discipline that involves the study of the chemical, physical, geological, and biological processes and reactions that govern the composition of the natural environment (including the biosphere, the cryosphere, th ...
processes between the environment and living organisms in the biosphere. It includes movements of carbon between the atmosphere and terrestrial and marine ecosystems, as well as soils and seafloor sediments. The fast cycle includes annual cycles involving photosynthesis and decadal cycles involving vegetative growth and decomposition. The reactions of the fast carbon cycle to human activities will determine many of the more immediate impacts of climate change. Creative Commons Attribution 4.0 International License
.
 The slow cycle is illustrated in the diagram above on the right. It involves medium to long-term geochemical processes belonging to the
The slow cycle is illustrated in the diagram above on the right. It involves medium to long-term geochemical processes belonging to the rock cycle
The rock cycle is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium conditi ...
. The exchange between the ocean and atmosphere can take centuries, and the weathering
Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs ''in situ'' (on site, with little or no movement) ...
of rocks can take millions of years. Carbon in the ocean precipitates to the ocean floor where it can form sedimentary rock
Sedimentary rocks are types of rock that are formed by the accumulation or deposition of mineral or organic particles at Earth's surface, followed by cementation. Sedimentation is the collective name for processes that cause these particles ...
and be subducted into the earth's mantle
Earth's mantle is a layer of silicate rock between the crust and the outer core. It has a mass of 4.01 × 1024 kg and thus makes up 67% of the mass of Earth. It has a thickness of making up about 84% of Earth's volume. It is predominantly so ...
. Mountain building
Mountain formation refers to the geological processes that underlie the formation of mountains. These processes are associated with large-scale movements of the Earth's crust (tectonic plates). Folding, faulting, volcanic activity, igneous int ...
processes result in the return of this geologic carbon to the Earth's surface. There the rocks are weathered and carbon is returned to the atmosphere by degassing
Degassing, also known as degasification, is the removal of dissolved gases from liquids, especially water or aqueous solutions. There are numerous methods for removing gases from liquids.
Gases are removed for various reasons. Chemists remove ga ...
and to the ocean by rivers. Other geologic carbon returns to the ocean through the hydrothermal emission of calcium ions. In a given year between 10 and 100 million tonnes of carbon moves around this slow cycle. This includes volcanoes returning geologic carbon directly to the atmosphere in the form of carbon dioxide. However, this is less than one percent of the carbon dioxide put into the atmosphere by burning fossil fuels.
Deep cycles
The terrestrial subsurface is the largest reservoir of carbon on earth, containing 14–135 Pg of carbon and 2–19% of all biomass. Microorganisms drive organic and inorganic compound transformations in this environment and thereby control biogeochemical cycles. Current knowledge of the microbial ecology of the subsurface is primarily based on16S ribosomal RNA
16 S ribosomal RNA (or 16 S rRNA) is the RNA component of the 30S subunit of a prokaryotic ribosome ( SSU rRNA). It binds to the Shine-Dalgarno sequence and provides most of the SSU structure.
The genes coding for it are referred to as 16S rR ...
(rRNA) gene sequences. Recent estimates show that <8% of 16S rRNA sequences in public databases derive from subsurface organisms and only a small fraction of those are represented by genomes or isolates. Thus, there is remarkably little reliable information about microbial metabolism in the subsurface. Further, little is known about how organisms in subsurface ecosystems are metabolically interconnected. Some cultivation-based studies of syntrophic consortia
A consortium (plural: consortia) is an association of two or more individuals, companies, organizations or governments (or any combination of these entities) with the objective of participating in a common activity or pooling their resources for ...
and small-scale metagenomic analyses of natural communities suggest that organisms are linked via metabolic handoffs: the transfer of redox reaction products of one organism to another. However, no complex environments have been dissected completely enough to resolve the metabolic interaction networks that underpin them. This restricts the ability of biogeochemical models to capture key aspects of the carbon and other nutrient cycles. New approaches such as genome-resolved metagenomics, an approach that can yield a comprehensive set of draft and even complete genomes for organisms without the requirement for laboratory isolation have the potential to provide this critical level of understanding of biogeochemical processes. Creative Commons Attribution 4.0 International License
.
Some examples
Some of the more well-known biogeochemical cycles are shown below:Carbon cycle
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major componen ...
File:Oxygen Cycle.jpg, Oxygen cycle
Oxygen cycle refers to the movement of oxygen through the atmosphere (air), biosphere (plants and animals) and the lithosphere (the Earth’s crust). The oxygen cycle demonstrates how free oxygen is made available in each of these regions, as wel ...
File:Nitrogen_Cycle.jpg, alt=Diagram of the nitrogen cycle, Nitrogen cycle
File:WhalePump.jpg, alt=Diagram of the nutrient cycle, Nutrient cycle
A nutrient cycle (or ecological recycling) is the movement and exchange of inorganic and organic matter back into the production of matter. Energy flow is a unidirectional and noncyclic pathway, whereas the movement of mineral nutrients is cyc ...
File:Phosphorus cycle.png, alt=Diagram of the phosphorus cycle, Phosphorus cycle
The phosphorus cycle is the biogeochemical cycle that describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the moveme ...
File:Sulfur Cycle (Ciclo do Enxofre).png, alt=Diagram of the sulfur cycle, Sulfur cycle
File:Rockcycle.jpg, alt=Diagram of the rock cycle, Rock cycle
The rock cycle is a basic concept in geology that describes transitions through geologic time among the three main rock types: sedimentary, metamorphic, and igneous. Each rock type is altered when it is forced out of its equilibrium conditi ...
File:Water cycle.png, alt=Diagram of the water cycle, Water cycle
The water cycle, also known as the hydrologic cycle or the hydrological cycle, is a biogeochemical cycle that describes the continuous movement of water on, above and below the surface of the Earth. The mass of water on Earth remains fairly cons ...
Climate change
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to ...
and human impacts are drastically changing the speed, intensity, and balance of these relatively unknown cycles, which include:
* the mercury cycle, and
* the human-caused cycle of PCBs.
Chloroplasts
A chloroplast () is a type of membrane-bound organelle known as a plastid that conducts photosynthesis mostly in plant and algal cells. The photosynthetic pigment chlorophyll captures the energy from sunlight, converts it, and stores it in ...
conduct photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored i ...
in plant cells and other eukaryotic
Eukaryotes () are organisms whose Cell (biology), cells have a cell nucleus, nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the ...
organisms.
File:Organic carbon cycle including the flow of kerogen.png, Kerogen
Kerogen is solid, insoluble organic matter in sedimentary rocks. Comprising an estimated 1016 tons of carbon, it is the most abundant source of organic compounds on earth, exceeding the total organic content of living matter 10,000-fold. It ...
cycle
File:Coal anthracite.jpg, Coal is a reservoir of carbon
ecology
Ecology () is the study of the relationships between living organisms, including humans, and their physical environment. Ecology considers organisms at the individual, population, community, ecosystem, and biosphere level. Ecology overl ...
and atmospheric sciences. Biochemical dynamics would also be related to the fields of geology
Geology () is a branch of natural science concerned with Earth and other astronomical objects, the features or rocks of which it is composed, and the processes by which they change over time. Modern geology significantly overlaps all other Ea ...
and pedology
Pedology (from Greek: πέδον, ''pedon'', "soil"; and λόγος, ''logos'', "study") is a discipline within soil science which focuses on understanding and characterizing soil formation, evolution, and the theoretical frameworks for modeling ...
.
See also
*Carbonate–silicate cycle
The carbonate–silicate geochemical cycle, also known as the inorganic carbon cycle, describes the long-term transformation of silicate rocks to carbonate rocks by weathering and sedimentation, and the transformation of carbonate rocks back int ...
* Ecological recycling
* Great Acceleration
* Hydrogen cycle
* Redox gradient
A redox gradient is a series of reduction-oxidation (redox) reactions sorted according to redox potential. The redox ladder displays the order in which redox reactions occur based on the free energy gained from redox pairs. These redox gradients ...
References
Further reading
*Schink, Bernhard; "Microbes: Masters of the Global Element Cycles" pp 33–58. "Metals, Microbes and Minerals: The Biogeochemical Side of Life", pp xiv + 341. Walter de Gruyter, BerlinDOI 10.1515/9783110589771-002
* * * * {{DEFAULTSORT:Biogeochemical Cycle Geochemistry Biogeography