Binding Site on:
[Wikipedia]
[Google]
[Amazon]
 In biochemistry and molecular biology, a binding site is a region on a
In biochemistry and molecular biology, a binding site is a region on a
 Enzymes incur catalysis by binding more strongly to transition states than substrates and products. At the catalytic binding site, several different interactions may act upon the substrate. These range from electric catalysis, acid and base catalysis, covalent catalysis, and metal ion catalysis. These interactions decrease the activation energy of a chemical reaction by providing favorable interactions to stabilize the high energy molecule. Enzyme binding allows for closer proximity and exclusion of substances irrelevant to the reaction. Side reactions are also discouraged by this specific binding.
Types of enzymes that can perform these actions include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases.
For instance, the transferase hexokinase catalyzes the phosphorylation of glucose to make glucose-6-phosphate. Active site residues of hexokinase allow for stabilization of the glucose molecule in the active site and spur the onset of an alternative pathway of favorable interactions, decreasing the activation energy.
Enzymes incur catalysis by binding more strongly to transition states than substrates and products. At the catalytic binding site, several different interactions may act upon the substrate. These range from electric catalysis, acid and base catalysis, covalent catalysis, and metal ion catalysis. These interactions decrease the activation energy of a chemical reaction by providing favorable interactions to stabilize the high energy molecule. Enzyme binding allows for closer proximity and exclusion of substances irrelevant to the reaction. Side reactions are also discouraged by this specific binding.
Types of enzymes that can perform these actions include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases.
For instance, the transferase hexokinase catalyzes the phosphorylation of glucose to make glucose-6-phosphate. Active site residues of hexokinase allow for stabilization of the glucose molecule in the active site and spur the onset of an alternative pathway of favorable interactions, decreasing the activation energy.
 Binding curves describe the binding behavior of ligand to a protein. Curves can be characterized by their shape, sigmoidal or hyperbolic, which reflect whether or not the protein exhibits
Binding curves describe the binding behavior of ligand to a protein. Curves can be characterized by their shape, sigmoidal or hyperbolic, which reflect whether or not the protein exhibits
 In the scope of cancer, ligands that are edited to have a similar appearance to the natural ligand are used to inhibit tumor growth. For example,
In the scope of cancer, ligands that are edited to have a similar appearance to the natural ligand are used to inhibit tumor growth. For example,
Drawing the active site of an enzyme
{{DEFAULTSORT:Binding Site Chemical bonding Structural biology Protein structure
 In biochemistry and molecular biology, a binding site is a region on a
In biochemistry and molecular biology, a binding site is a region on a macromolecule
A macromolecule is a very large molecule important to biophysical processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The ...
such as a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
. Ligands may include other proteins (resulting in a protein-protein interaction), enzyme substrates, second messengers
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. (Intercellular signals, a non-local form or cell signaling, encompassing both first m ...
, hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required ...
s, or allosteric modulator
In pharmacology and biochemistry, allosteric modulators are a group of substances that bind to a receptor to change that receptor's response to stimulus. Some of them, like benzodiazepines, are drugs. The site that an allosteric modulator binds to ...
s. The binding event is often, but not always, accompanied by a conformational change
In biochemistry, a conformational change is a change in the shape of a macromolecule, often induced by environmental factors.
A macromolecule is usually flexible and dynamic. Its shape can change in response to changes in its environment or oth ...
that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. Th ...
), but can also be covalent reversible or irreversible.
Function
Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts ofsignal transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellula ...
pathways. Types of ligands include neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neu ...
s, toxin
A toxin is a naturally occurring organic poison produced by metabolic activities of living cells or organisms. Toxins occur especially as a protein or conjugated protein. The term toxin was first used by organic chemist Ludwig Brieger (1849 ...
s, neuropeptide
Neuropeptides are chemical messengers made up of small chains of amino acids that are synthesized and released by neurons. Neuropeptides typically bind to G protein-coupled receptors (GPCRs) to modulate neural activity and other tissues like t ...
s, and steroid hormone
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Withi ...
s. Binding sites incur functional changes in a number of contexts, including enzyme catalysis, molecular pathway signaling, homeostatic regulation, and physiological function. Electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons res ...
, steric shape and geometry of the site selectively allow for highly specific ligands to bind, activating a particular cascade of cellular interactions the protein is responsible for.
Catalysis
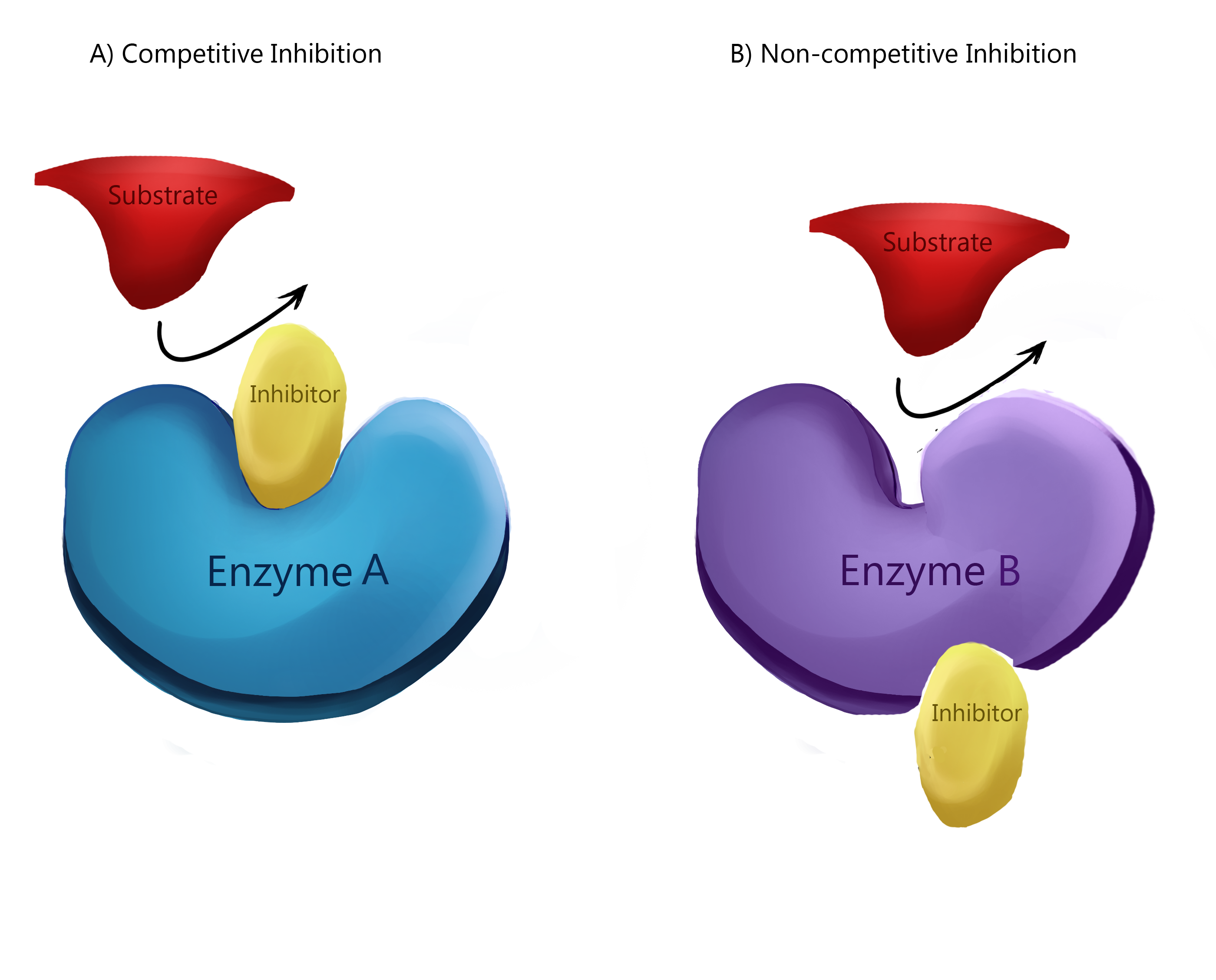
Inhibition
Protein inhibition by inhibitor binding may induce obstruction in pathway regulation, homeostatic regulation and physiological function. Competitive inhibitors compete with substrate to bind to free enzymes at active sites and thus impede the production of the enzyme-substrate complex upon binding. For example, carbon monoxide poisoning is caused by the competitive binding of carbon monoxide as opposed to oxygen in hemoglobin. Uncompetitive inhibitors, alternatively, bind concurrently with substrate at active sites. Upon binding to an enzyme substrate (ES) complex, an enzyme substrate inhibitor (ESI) complex is formed. Similar to competitive inhibitors, the rate at product formation is decreased also. Lastly, mixed inhibitors are able to bind to both the free enzyme and the enzyme-substrate complex. However, in contrast to competitive and uncompetitive inhibitors, mixed inhibitors bind to the allosteric site. Allosteric binding induces conformational changes that may increase the protein's affinity for substrate. This phenomenon is called positive modulation. Conversely, allosteric binding that decreases the protein's affinity for substrate is negative modulation.Types
Active site
At the active site, a substrate binds to an enzyme to induce a chemical reaction. Substrates, transition states, and products can bind to the active site, as well as any competitive inhibitors. For example, in the context of protein function, the binding of calcium to troponin in muscle cells can induce a conformational change in troponin. This allows for tropomyosin to expose the actin-myosin binding site to which the myosin head binds to form a cross-bridge and induce amuscle contraction
Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such a ...
.
In the context of the blood, an example of competitive binding is carbon monoxide which competes with oxygen for the active site on heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
. Carbon monoxide's high affinity may outcompete oxygen in the presence of low oxygen concentration. In these circumstances, the binding of carbon monoxide induces a conformation change that discourages heme from binding to oxygen, resulting in carbon monoxide poisoning.

Allosteric site
At the regulatory site, the binding of a ligand may elicit amplified or inhibited protein function. The binding of a ligand to an allosteric site of a multimeric enzyme often induces positive cooperativity, that is the binding of one substrate induces a favorable conformation change and increases the enzyme's likelihood to bind to a second substrate. Regulatory site ligands can involve homotropic and heterotropic ligands, in which single or multiple types of molecule affects enzyme activity respectively. Enzymes that are highly regulated are often essential in metabolic pathways. For example, phosphofructokinase (PFK), which phosphorylates fructose in glycolysis, is largely regulated by ATP. Its regulation in glycolysis is imperative because it is the committing and rate limiting step of the pathway. PFK also controls the amount of glucose designated to form ATP through thecatabolic
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lip ...
pathway. Therefore, at sufficient levels of ATP, PFK is allosterically inhibited by ATP. This regulation efficiently conserves glucose reserves, which may be needed for other pathways. Citrate, an intermediate of the citric acid cycle, also works as an allosteric regulator of PFK.
Single- and multi-chain binding sites
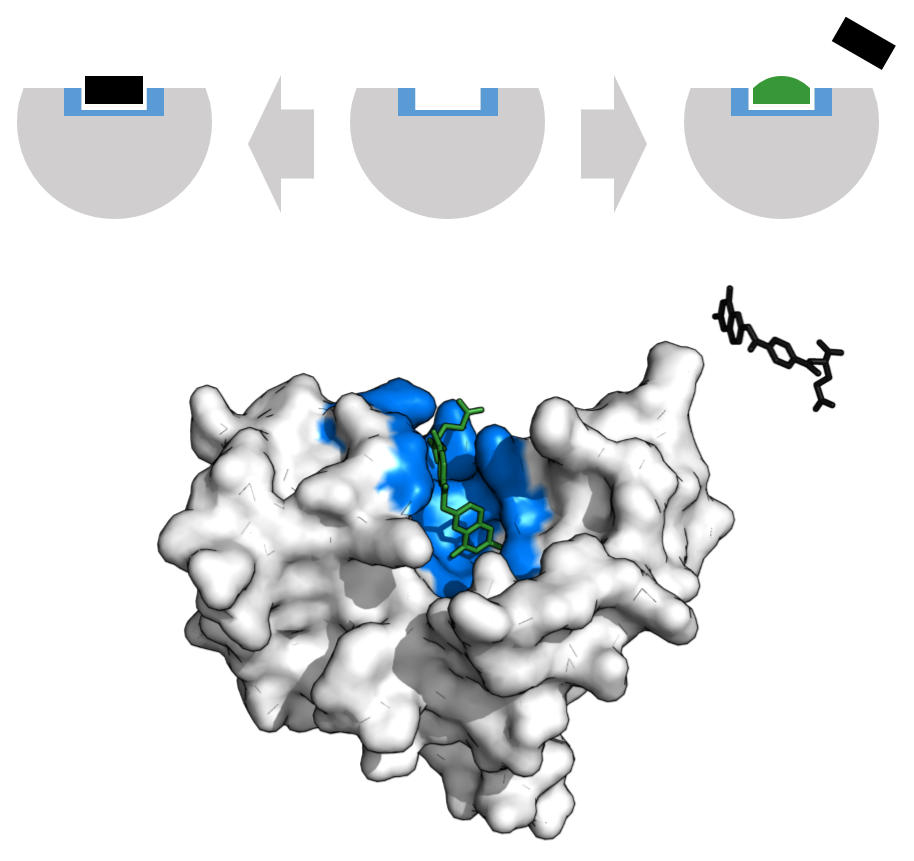
Binding sites can be characterized also by their structural features. Single-chain sites (of “monodesmic” ligands, μόνος: single, δεσμός: binding) are formed by a single protein chain, while multi-chain sites (of "polydesmic” ligands, πολοί: many) are frequent in protein complexes, and are formed by ligands that bind more than one protein chain, typically in or near protein interfaces. Recent research shows that binding site structure has profound consequences for the biology of protein complexes (evolution of function, allostery).Cryptic binding sites
Cryptic binding sites are the binding sites that are transiently formed in an apo form or that are induced by ligand binding. Considering the cryptic binding sites increases the size of the potentially “ druggable” human proteome from ~40% to ~78% of disease-associated proteins. The binding sites have been investigated by:support vector machine
In machine learning, support vector machines (SVMs, also support vector networks) are supervised learning models with associated learning algorithms that analyze data for classification and regression analysis. Developed at AT&T Bell Laborat ...
applied to "CryptoSite" data set, Extension of "CryptoSite" data set, long timescale molecular dynamics simulation with Markov state model and with biophysical experiments, and cryptic-site index that is based on relative accessible surface area
The accessible surface area (ASA) or solvent-accessible surface area (SASA) is the surface area of a biomolecule that is accessible to a solvent. Measurement of ASA is usually described in units of square angstroms (a standard unit of measurement ...
.
Binding curves
cooperative
A cooperative (also known as co-operative, co-op, or coop) is "an autonomous association of persons united voluntarily to meet their common economic, social and cultural needs and aspirations through a jointly owned and democratically-contro ...
or noncooperative binding behavior respectively. Typically, the x-axis describes the concentration of ligand and the y-axis describes the fractional saturation of ligands bound to all available binding sites. The Michaelis Menten equation is usually used when determining the shape of the curve. The Michaelis Menten equation is derived based on steady-state conditions and accounts for the enzyme reactions taking place in a solution. However, when the reaction takes place while the enzyme is bound to a substrate, the kinetics play out differently.
Modeling with binding curves are useful when evaluating the binding affinities of oxygen to hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythroc ...
and myoglobin
Myoglobin (symbol Mb or MB) is an iron- and oxygen-binding protein found in the cardiac and skeletal muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin. Compared to hemoglobin, myoglob ...
in the blood. Hemoglobin, which has four heme groups, exhibits cooperative binding
Molecular binding is an interaction between molecules that results in a stable physical association between those molecules. Cooperative binding occurs in binding systems containing more than one type, or species, of molecule and in which one of t ...
. This means that the binding of oxygen to a heme group on hemoglobin induces a favorable conformation change that allows for increased binding favorability of oxygen for the next heme groups. In these circumstances, the binding curve of hemoglobin will be sigmoidal due to its increased binding favorability for oxygen. Since myoglobin has only one heme group, it exhibits noncooperative binding which is hyperbolic on a binding curve.
Applications
Biochemical differences between different organisms and humans are useful for drug development. For instance,penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from '' Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum usin ...
kills bacteria by inhibiting the bacterial enzyme DD-transpeptidase
DD-transpeptidase (, ''DD-peptidase'', ''DD-transpeptidase'', ''DD-carboxypeptidase'', ''D-alanyl-D-alanine carboxypeptidase'', ''D-alanyl-D-alanine-cleaving-peptidase'', ''D-alanine carboxypeptidase'', ''D-alanyl carboxypeptidase'', and ''serine-t ...
, destroying the development of the bacterial cell wall and inducing cell death. Thus, the study of binding sites is relevant to many fields of research, including cancer mechanisms, drug formulation, and physiological regulation. The formulation of an inhibitor to mute a protein's function is a common form of pharmaceutical therapy.
 In the scope of cancer, ligands that are edited to have a similar appearance to the natural ligand are used to inhibit tumor growth. For example,
In the scope of cancer, ligands that are edited to have a similar appearance to the natural ligand are used to inhibit tumor growth. For example, Methotrexate
Methotrexate (MTX), formerly known as amethopterin, is a chemotherapy agent and immune-system suppressant. It is used to treat cancer, autoimmune diseases, and ectopic pregnancies. Types of cancers it is used for include breast cancer, leuke ...
, a chemotherapeutic
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherap ...
, acts as a competitive inhibitor at the dihydrofolate reductase
Dihydrofolate reductase, or DHFR, is an enzyme that reduces dihydrofolic acid to tetrahydrofolic acid, using NADPH as an electron donor, which can be converted to the kinds of tetrahydrofolate cofactors used in 1-carbon transfer chemistry ...
active site. This interaction inhibits the synthesis of tetrahydrofolate, shutting off production of DNA, RNA and proteins. Inhibition of this function represses neoplastic growth and improves severe psoriasis
Psoriasis is a long-lasting, noncontagious autoimmune disease characterized by raised areas of abnormal skin. These areas are red, pink, or purple, dry, itchy, and scaly. Psoriasis varies in severity from small, localized patches to comple ...
and adult rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are inv ...
.
In cardiovascular illnesses, drugs such as beta blockers are used to treat patients with hypertension. Beta blocker
Beta blockers, also spelled β-blockers, are a class of medications that are predominantly used to manage abnormal heart rhythms, and to protect the heart from a second heart attack after a first heart attack ( secondary prevention). They are ...
s (β-Blockers) are antihypertensive agents that block the binding of the hormones adrenaline and noradrenaline to β1 and β2 receptors in the heart and blood vessels. These receptors normally mediate the sympathetic "fight or flight" response, causing constriction of the blood vessels.
Competitive inhibitors are also largely found commercially. Botulinum toxin
Botulinum toxin, or botulinum neurotoxin (BoNT), is a neurotoxic protein produced by the bacterium ''Clostridium botulinum'' and related species. It prevents the release of the neurotransmitter acetylcholine from axon endings at the neurom ...
, known commercially as Botox, is a neurotoxin causes flaccid paralysis in the muscle due to binding to acetylcholine dependent nerves. This interaction inhibits muscle contractions, giving the appearance of smooth muscle.
Prediction
A number of computational tools have been developed for the prediction of the location of binding sites on proteins. These can be broadly classified into sequence based or structure based. Sequence based methods rely on the assumption that the sequences of functionally conserved portions of proteins such as binding site are conserved. Structure based methods require the 3D structure of the protein. These methods in turn can be subdivided into template and pocket based methods. Template based methods search for 3D similarities between the target protein and proteins with known binding sites. The pocket based methods search for concave surfaces or buried pockets in the target protein that possess features such ashydrophobicity
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
and hydrogen bonding capacity that would allow them to bind ligands with high affinity. Even though the term pocket is used here, similar methods can be used to predict binding sites used in protein-protein interactions that are usually more planar, not in pockets.
References
External links
*Drawing the active site of an enzyme
{{DEFAULTSORT:Binding Site Chemical bonding Structural biology Protein structure