Atomic Radii on:
[Wikipedia]
[Google]
[Amazon]
 The atomic radius of a
The atomic radius of a  For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the
For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the
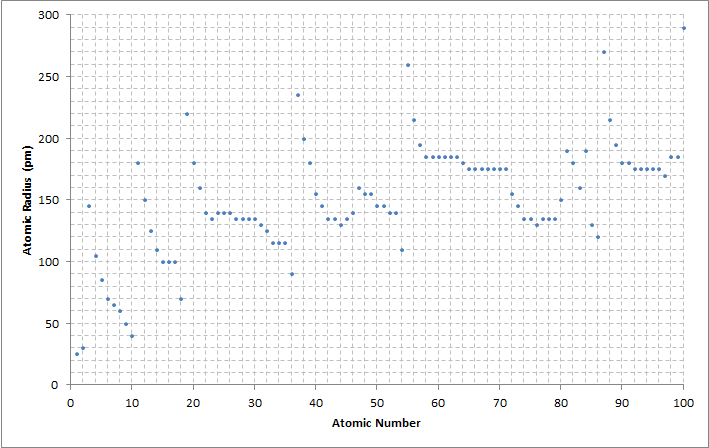 The way the atomic radius varies with increasing
The way the atomic radius varies with increasing
La3+ > Ce3+ > ..., ... > Lu3+. # There is a regular decrease in their ionic radii. # There is a regular decrease in their tendency to act as a reducing agent, with an increase in atomic number. # The second and third rows of d-block transition elements are quite close in properties. # Consequently, these elements occur together in natural minerals and are difficult to separate.
chemical element
A chemical element is a species of atoms that have a given number of protons in their nuclei, including the pure substance consisting only of that species. Unlike chemical compounds, chemical elements cannot be broken down into simpler sub ...
is a measure of the size of its atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, ...
, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Four widely used definitions of atomic radius are: Van der Waals radius
The van der Waals radius, ''r'', of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom.
It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, ...
, ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the catio ...
, metallic radius and covalent radius
The covalent radius, ''r''cov, is a measure of the size of an atom that forms part of one covalent bond. It is usually measured either in picometres (pm) or angstroms (Å), with 1 Å = 100 pm.
In principle, the sum of the two coval ...
. Typically, because of the difficulty to isolate atoms in order to measure their radii separately, atomic radius is measured in a chemically bonded state; however theoretical calculations are simpler when considering atoms in isolation. The dependencies on environment, probe, and state lead to a multiplicity of definitions.
Depending on the definition, the term may apply to atoms in condensed matter
Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid phases which arise from electromagnetic forces between atoms. More generally, the su ...
, covalently bonding in molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
s, or in ionized
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule ...
and excited states; and its value may be obtained through experimental measurements, or computed from theoretical models. The value of the radius may depend on the atom's state and context.
Electrons do not have definite orbits nor sharply defined ranges. Rather, their positions must be described as probability distributions that taper off gradually as one moves away from the nucleus, without a sharp cutoff; these are referred to as atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in an ...
s or electron clouds. Moreover, in condensed matter and molecules, the electron clouds of the atoms usually overlap to some extent, and some of the electrons may roam over a large region encompassing two or more atoms.
Under most definitions the radii of isolated neutral atoms range between 30 and 300 pm ( trillionths of a meter), or between 0.3 and 3 ångström
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
s. Therefore, the radius of an atom is more than 10,000 times the radius of its nucleus (1–10 fm),
and less than 1/1000 of the wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, t ...
of visible light
Light or visible light is electromagnetic radiation that can be perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400–700 nanometres (nm), corresponding to frequencies of 750–420 te ...
(400–700 nm).
 For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the
For many purposes, atoms can be modeled as spheres. This is only a crude approximation, but it can provide quantitative explanations and predictions for many phenomena, such as the density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
of liquids and solids, the diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemica ...
of fluids through molecular sieves, the arrangement of atoms and ions in crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macro ...
s, and the size and shape of molecules.
History
In 1920, shortly after it had become possible to determine the sizes of atoms usingX-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
, it was suggested that all atoms of the same element have the same radii. However, in 1923, when more crystal data had become available, it was found that the approximation of an atom as a sphere does not necessarily hold when comparing the same atom in different crystal structures.
Definitions
Widely used definitions of atomic radius include: *Van der Waals radius
The van der Waals radius, ''r'', of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom.
It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, ...
: In the simplest definition, half the minimum distance between the nuclei of two atoms of the element that are not otherwise bound by covalent or metallic interactions.
The Van der Waals radius may be defined even for elements (such as metals) in which Van der Waals forces are dominated by other interactions. Because Van der Waals interactions arise through quantum fluctuations of the atomic polarisation, the polarisability (which can usually be measured or calculated more easily) may be used to define the Van der Waals radius indirectly.
* Ionic radius
Ionic radius, ''r''ion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the catio ...
: the nominal radius of the ions of an element in a specific ionization state, deduced from the spacing of atomic nuclei in crystalline salts that include that ion. In principle, the spacing between two adjacent oppositely charged ions (the length of the ionic bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds ...
between them) should equal the sum of their ionic radii.
* Covalent radius
The covalent radius, ''r''cov, is a measure of the size of an atom that forms part of one covalent bond. It is usually measured either in picometres (pm) or angstroms (Å), with 1 Å = 100 pm.
In principle, the sum of the two coval ...
: the nominal radius of the atoms of an element when covalently bound to other atoms, as deduced from the separation between the atomic nuclei in molecules. In principle, the distance between two atoms that are bound to each other in a molecule (the length of that covalent bond) should equal the sum of their covalent radii.
* Metallic radius: the nominal radius of atoms of an element when joined to other atoms by metallic bond
Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be des ...
s.
* Bohr radius
The Bohr radius (''a''0) is a physical constant, approximately equal to the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an ...
: the radius of the lowest-energy electron orbit predicted by Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar Syst ...
of the atom (1913).
It is only applicable to atoms and ions with a single electron, such as hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
, singly ionized helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. ...
, and positronium
Positronium (Ps) is a system consisting of an electron and its anti-particle, a positron, bound together into an exotic atom, specifically an onium. Unlike hydrogen, the system has no protons. The system is unstable: the two particles annih ...
. Although the model itself is now obsolete, the Bohr radius for the hydrogen atom is still regarded as an important physical constant.
Empirically measured atomic radius
The following table shows empirically measured covalent radii for the elements, as published by J. C. Slater in 1964. The values are in picometers (pm or 1×10−12 m), with an accuracy of about 5 pm. The shade of the box ranges from red to yellow as the radius increases; gray indicates lack of data.Explanation of the general trends
 The way the atomic radius varies with increasing
The way the atomic radius varies with increasing atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
can be explained by the arrangement of electrons in shells of fixed capacity. The shells are generally filled in order of increasing radius, since the negatively charged electrons are attracted by the positively charged protons in the nucleus. As the atomic number increases along each row of the periodic table, the additional electrons go into the same outermost shell; whose radius gradually contracts, due to the increasing nuclear charge. In a noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
, the outermost shell is completely filled; therefore, the additional electron of next alkali metal will go into the next outer shell, accounting for the sudden increase in the atomic radius.
The increasing nuclear charge is partly counterbalanced by the increasing number of electrons, a phenomenon that is known as shielding; which explains why the size of atoms usually increases down each column. However, there is one notable exception, known as the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii ...
: the 5d block of elements are much smaller than one would expect, due to the weak shielding of the 4f electrons.
Essentially, the atomic radius decreases across the periods due to an increasing number of protons. Therefore, there is a greater attraction between the protons and electrons because opposite charges attract, and more protons create a stronger charge. The greater attraction draws the electrons closer to the protons, decreasing the size of the particle. Therefore, the atomic radius decreases. Down the groups, atomic radius increases. This is because there are more energy levels and therefore a greater distance between protons and electrons. In addition, electron shielding causes attraction to decrease, so remaining electrons can go farther away from the positively charged nucleus. Therefore, the size, or atomic radius, increases.
The following table summarizes the main phenomena that influence the atomic radius of an element:
Lanthanide contraction
The electrons in the 4f- subshell, which is progressively filled from lanthanum ('' Z'' = 57) toytterbium
Ytterbium is a chemical element with the symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. However, like the othe ...
(''Z'' = 70), are not particularly effective at shielding the increasing nuclear charge from the sub-shells further out. The elements immediately following the lanthanides have atomic radii which are smaller than would be expected and which are almost identical to the atomic radii of the elements immediately above them.
Hence lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
is in fact slightly smaller than yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a " rare-earth element". Yttrium is almost always found in co ...
, hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri M ...
has virtually the same atomic radius (and chemistry) as zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
, and tantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as ''tantalium'', it is named after Tantalus, a villain in Greek mythology. Tantalum is a very hard, ductile, lustrous, blue-gray transition metal that ...
has an atomic radius similar to niobium, and so forth. The effect of the lanthanide contraction is noticeable up to platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
(''Z'' = 78), after which it is masked by a relativistic effect
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of ...
known as the inert pair effect The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of ox ...
.
Due to lanthanide contraction, the 5 following observations can be drawn:
# The size of Ln3+ ions regularly decreases with atomic number. According to Fajans' rules, decrease in size of Ln3+ ions increases the covalent character and decreases the basic character between Ln3+ and OH− ions in Ln(OH)3, to the point that Yb(OH)3 and Lu(OH)3 can dissolve with difficulty in hot concentrated NaOH. Hence the order of size of Ln3+ is given: La3+ > Ce3+ > ..., ... > Lu3+. # There is a regular decrease in their ionic radii. # There is a regular decrease in their tendency to act as a reducing agent, with an increase in atomic number. # The second and third rows of d-block transition elements are quite close in properties. # Consequently, these elements occur together in natural minerals and are difficult to separate.
d-block contraction
The d-block contraction is less pronounced than the lanthanide contraction but arises from a similar cause. In this case, it is the poor shielding capacity of the 3d-electrons which affects the atomic radii and chemistries of the elements immediately following the first row of thetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that ca ...
s, from gallium (''Z'' = 31) to bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
(''Z'' = 35).
Calculated atomic radius
The following table shows atomic radii computed from theoretical models, as published by Enrico Clementi and others in 1967. The values are in picometres (pm).See also
* Atomic radii of the elements (data page) *Chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
*Covalent radius
The covalent radius, ''r''cov, is a measure of the size of an atom that forms part of one covalent bond. It is usually measured either in picometres (pm) or angstroms (Å), with 1 Å = 100 pm.
In principle, the sum of the two coval ...
*Bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
*Steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
* Kinetic diameter
Notes
* Difference between empirical and calculated data: Empirical data means "originating in or based on observation or experience" or "relying on experience or observation alone often without due regard for system and theory data". In other words, the data are measured through physical observation, and vetted by other experiments generating ''similar results''. Calculated data, on the other hand, are derived from theoretical models. Such predictions are especially useful for elements whose radii cannot be measured experimentally (e.g. those that have not been discovered, or that have too short of a half-life).References
{{DEFAULTSORT:Atomic Radius Atomic radius Properties of chemical elements