Aromatic ring on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, aromaticity is a chemical property of
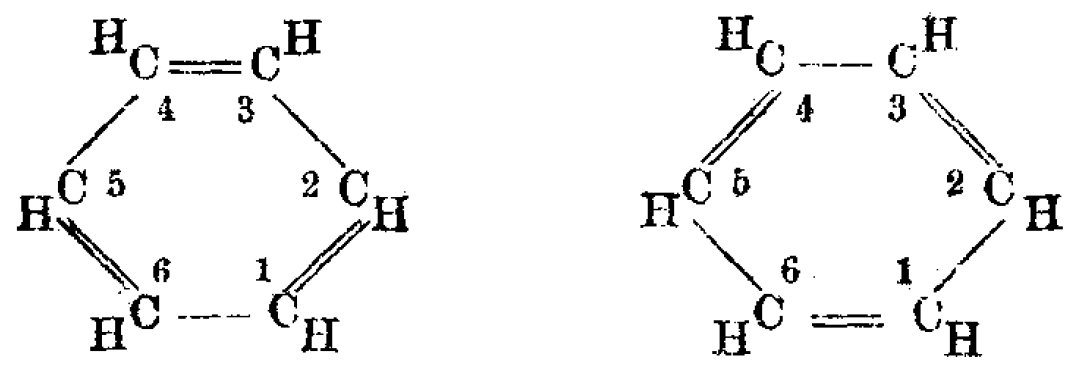
 In the 19th century, chemists found it puzzling that benzene could be so unreactive toward addition reactions, given its presumed high degree of unsaturation. The cyclohexatriene structure for
In the 19th century, chemists found it puzzling that benzene could be so unreactive toward addition reactions, given its presumed high degree of unsaturation. The cyclohexatriene structure for
cyclic
Cycle, cycles, or cyclic may refer to:
Anthropology and social sciences
* Cyclic history, a theory of history
* Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr.
* Social cycle, various cycles in s ...
( ring-shaped), ''typically'' planar
Planar is an adjective meaning "relating to a plane (geometry)".
Planar may also refer to:
Science and technology
* Planar (computer graphics), computer graphics pixel information from several bitplanes
* Planar (transmission line technologies), ...
(flat) molecular structures with pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals ...
s in resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied Periodic function, periodic force (or a Fourier analysis, Fourier component of it) is equal or close to a natural frequency of the system ...
(those containing delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
s) that gives increased stability compared to saturated compound
In chemistry, a saturated compound is a chemical compound (or ion) that resists the addition reactions, such as hydrogenation, oxidative addition, and binding of a Lewis base. The term is used in many contexts and for many classes of chemical ...
s having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. T ...
s that are not aromatic are classified as aliphatic compounds—they might be cyclic
Cycle, cycles, or cyclic may refer to:
Anthropology and social sciences
* Cyclic history, a theory of history
* Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr.
* Social cycle, various cycles in s ...
, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma
An odor (American English) or odour ( Commonwealth English; see spelling differences) is caused by one or more volatilized chemical compounds that are generally found in low concentrations that humans and animals can perceive via their se ...
, but is a distinct property from that meaning.
Since the most common aromatic compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping ...
s are derivatives of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
(an aromatic hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
common in petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases (Purine) in RNA and DNA. An aromatic functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
or other substituent is called an aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromaticity, aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar ...
group.
In terms of the electronic nature of the molecule, aromaticity describes a conjugated system often represented in Lewis diagrams
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons th ...
as alternating single and double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
s in a ring. In reality, the electrons represented by the double bonds in the Lewis diagram are actually distributed evenly around the ring ("delocalized"), increasing the molecule's stability. Due to the restrictions imposed by the way Lewis diagrams are drawn, the molecule cannot be represented by one diagram, but rather a hybrid of multiple different diagrams (called resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied Periodic function, periodic force (or a Fourier analysis, Fourier component of it) is equal or close to a natural frequency of the system ...
), such as with the two resonance structures of benzene. These molecules cannot be found in either one of these representations, with the longer single bonds in one location and the shorter double bond in another (see below). Rather, the molecule exhibits all equal bond lengths in between those of single and double bonds. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé
Friedrich August Kekulé, later Friedrich August Kekule von Stradonitz ( , ; 7 September 1829 – 13 July 1896), was a German organic chemist. From the 1850s until his death, Kekulé was one of the most prominent chemists in Europe, especially ...
(see below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.
Theory
As it is a standard for resonance diagrams, the use of a double-headed arrow indicates that two structures are not distinct entities but merely hypothetical possibilities. Neither is an accurate representation of the ''actual'' compound, which is best represented by a hybrid (average) of these structures. A C=C bond is shorter than a C−C bond. Benzene is a regular hexagon—it is planar and all six carbon–carbon bonds have the same length, which is intermediate between that of a single and that of adouble bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betwee ...
.
In a cyclic molecule with three alternating double bonds, cyclohexatriene, the bond length of the single bond would be 1.54 Å and that of the double bond would be 1.34 Å. However, in a molecule of benzene, the length of each of the bonds is 1.40 Å, indicating it to be the average of single and double bond.
A better representation is that of the circular π-bond (Armstrong's ''inner cycle''), in which the electron density is evenly distributed through a π-bond above and below the ring. This model more correctly represents the location of electron density within the aromatic ring.
The single bonds are formed from overlap of hybridized atomic sp2-orbitals in line between the carbon nuclei—these are called σ-bonds. Double bonds consist of a σ-bond and a π-bond. The π-bonds are formed from overlap of atomic p-orbitals above and below the plane of the ring. The following diagram shows the positions of these p-orbitals:
:
Since they are out of the plane of the atoms, these orbitals can interact with each other freely, and become delocalized. This means that, instead of being tied to one atom of carbon, each electron is shared by all six in the ring. Thus, there are not enough electrons to form double bonds on all the carbon atoms, but the "extra" electrons strengthen all of the bonds on the ring equally. The resulting molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of findin ...
is considered to have ''π symmetry''.
:
History
The term "aromatic"
The first known use of the word "aromatic" as a ''chemical'' term—namely, to apply to compounds that contain thephenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
group—occurs in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which have odors (aromas), unlike pure saturated hydrocarbons. Aromaticity as a chemical property bears no general relationship with the olfactory properties of such compounds (how they smell), although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plant
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclu ...
s, such as terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ...
s, had chemical properties that we recognize today are similar to unsaturated petroleum hydrocarbons like benzene. If this was indeed the earliest introduction of the term, it is curious that Hofmann says nothing about why he introduced an adjective indicating olfactory character to apply to a group of chemical substances, of which only some have notable aromas. Also, some of the most odoriferous organic substances known are terpenes
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes ar ...
, which are not aromatic in the chemical sense. Terpenes and benzenoid
In organic chemistry, benzenoids are a class of organic compounds with at least one benzene ring. These compounds have increased stability due resonance in the benzene rings. Most aromatic hydrocarbons are benzenoid. Notable counterexamples are cy ...
substances do have a chemical characteristic in common, that is, higher unsaturation than many aliphatic compounds, and Hofmann may not have made a distinction between the two categories. Many of the earliest-known examples of aromatic compounds, such as benzene and toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) a ...
, have distinctive pleasant smells. This property led to the term "aromatic" for this class of compounds, and hence the term "aromaticity" for the eventually discovered electronic property.
The structure of the benzene ring

benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
was first proposed by August Kekulé
Friedrich August Kekulé, later Friedrich August Kekule von Stradonitz ( , ; 7 September 1829 – 13 July 1896), was a German organic chemist. From the 1850s until his death, Kekulé was one of the most prominent chemists in Europe, especially ...
in 1865. Most chemists were quick to accept this structure, since it accounted for most of the known isomeric relationships of aromatic chemistry. The hexagonal structure explains why only one isomer of benzene exists and why disubstituted compounds have three isomers.
Between 1897 and 1906, J. J. Thomson
Sir Joseph John Thomson (18 December 1856 – 30 August 1940) was a British physicist and Nobel Laureate in Physics, credited with the discovery of the electron, the first subatomic particle to be discovered.
In 1897, Thomson showed that ...
, the discoverer of the electron, proposed three equivalent electrons between each pair of carbon atoms in benzene. An explanation for the exceptional stability of benzene is conventionally attributed to Sir Robert Robinson, who was apparently the first (in 1925) to coin the term ''aromatic sextet'' as a group of six electrons that resists disruption.
In fact, this concept can be traced further back, via Ernest Crocker in 1922, to Henry Edward Armstrong
Henry Edward Armstrong FRS FRSE (Hon) (6 May 1848 – 13 July 1937) was a British chemist. Although Armstrong was active in many areas of scientific research, such as the chemistry of naphthalene derivatives, he is remembered today largely for h ...
, who in 1890 wrote "the ixcentric affinities act within a cycle … benzene may be represented by a double ring … and when an additive compound is formed, the inner cycle of affinity suffers disruption, the contiguous carbon-atoms to which nothing has been attached of necessity acquire the ethylenic condition".
Here, Armstrong is describing at least four modern concepts. First, his "affinity" is better known nowadays as the electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
, which was to be discovered only seven years later by J. J. Thomson. Second, he is describing electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
, proceeding (third) through a Wheland intermediate, in which (fourth) the conjugation
Conjugation or conjugate may refer to:
Linguistics
* Grammatical conjugation, the modification of a verb from its basic form
* Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
* Complex conjugation, the chang ...
of the ring is broken. He introduced the symbol C centered on the ring as a shorthand for the ''inner cycle'', thus anticipating Erich Clar's notation. It is argued that he also anticipated the nature of wave mechanics Wave mechanics may refer to:
* the mechanics of waves
* the ''wave equation'' in quantum physics, see Schrödinger equation
See also
* Quantum mechanics
* Wave equation
The (two-way) wave equation is a second-order linear partial different ...
, since he recognized that his affinities had direction, not merely being point particles, and collectively having a distribution that could be altered by introducing substituents onto the benzene ring (much as the distribution of the electric charge in a body is altered by bringing it near to another body).
The quantum mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
origins of this stability, or aromaticity, were first modelled by Hückel in 1931. He was the first to separate the bonding electrons into sigma and pi electrons.
Aromaticity of an arbitrary aromatic compound can be measured quantitatively by the nucleus-independent chemical shift
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electro ...
(NICS) computational method and aromaticity percentage methods.
Characteristics of aromatic systems
An aromatic (oraryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromaticity, aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar ...
) ring contains a set of covalently bound atoms with specific characteristics:
# A delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
conjugated π system, most commonly an arrangement of alternating single and double bonds
# Coplanar
In geometry, a set of points in space are coplanar if there exists a geometric plane that contains them all. For example, three points are always coplanar, and if the points are distinct and non-collinear, the plane they determine is unique. How ...
structure, with all the contributing atoms in the same plane
# Contributing atoms arranged in one or more rings
# A number of π delocalized electrons that is even, but not a multiple of 4. That is, 4''n'' + 2 π-electrons, where ''n'' = 0, 1, 2, 3, and so on. This is known as Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was ...
.
According to Hückel's rule, if a molecule has 4''n'' + 2 π-electrons, it is aromatic, but if it has 4''n'' π-electrons and has characteristics 1–3 above, the molecule is said to be antiaromatic
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. Unlike aroma ...
. Whereas benzene is aromatic (6 electrons, from 3 double bonds), cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is ...
is antiaromatic, since the number of π delocalized electrons is 4, which of course is a multiple of 4. The cyclobutadienide(2−) ion, however, is aromatic (6 electrons). An atom in an aromatic system can have other electrons that are not part of the system, and are therefore ignored for the 4''n'' + 2 rule. In furan, the oxygen atom is sp2 hybridized. One lone pair is in the π system and the other in the plane of the ring (analogous to the C–H bond in the other positions). There are 6 π-electrons, so furan is aromatic.
Aromatic molecules typically display enhanced chemical stability, compared with similar non-aromatic molecules. A molecule that can be aromatic will tend to change toward aromaticity, and the added stability changes the chemistry of the molecule. Aromatic compounds undergo electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic n ...
and nucleophilic aromatic substitution
A nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compou ...
reactions, but not electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic Che ...
reactions as happens with carbon–carbon double bonds.
In the presence of a magnetic field, the circulating π-electrons in an aromatic molecule produce an aromatic ring current
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons ...
that induces an additional magnetic field, an important effect in nuclear magnetic resonance
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
. The NMR signal of protons in the plane of an aromatic ring are shifted substantially further down-field than those on non-aromatic sp2 carbons. This is an important way of detecting aromaticity. By the same mechanism, the signals of protons located near the ring axis are shifted upfield.
Aromatic molecules are able to interact with each other in so-called π–π stacking: The π systems form two parallel rings overlap in a "face-to-face" orientation. Aromatic molecules are also able to interact with each other in an "edge-to-face" orientation: The slight positive charge of the substituents on the ring atoms of one molecule are attracted to the slight negative charge of the aromatic system on another molecule.
Planar monocyclic molecules containing 4''n'' π-electrons are called antiaromatic
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. Unlike aroma ...
and are, in general, unstable. Molecules that could be antiaromatic
Antiaromaticity is a chemical property of a cyclic molecule with a π electron system that has higher energy, i.e., it is less stable due to the presence of 4n delocalised (π or lone pair) electrons in it, as opposed to aromaticity. Unlike aroma ...
will tend to change from this electronic or conformation, thereby becoming non-aromatic. For example, cyclooctatetraene
1,3,5,7-Cyclooctatetraene (COT) is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as nnulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature. Because of ...
(COT) distorts out of planarity, breaking π overlap between adjacent double bonds. Recent studies have determined that cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is ...
adopts an asymmetric, rectangular configuration in which single and double bonds indeed alternate, with no resonance; the single bonds are markedly longer than the double bonds, reducing unfavorable p-orbital overlap. This reduction of symmetry lifts the degeneracy of the two formerly non-bonding molecular orbitals, which by Hund's rule
Hund's rule of maximum multiplicity is a rule based on observation of atomic spectra, which is used to predict the ground state of an atom or molecule with one or more open electronic shells. The rule states that for a given electron configuration ...
forces the two unpaired electrons into a new, weakly bonding orbital (and also creates a weakly antibonding orbital). Hence, cyclobutadiene is non-aromatic; the strain of the asymmetric configuration outweighs the anti-aromatic destabilization that would afflict the symmetric, square configuration.
Hückel's rule of aromaticity treats molecules in their singlet ground states (S0). The stability trends of the compounds described here are found to be reversed in the lowest lying triplet and singlet excited states (T1 and S1), according to Baird's rule In organic chemistry, Baird's rule estimates whether the lowest triplet state of planar, cyclic structures will have aromatic properties or not. The quantum mechanical basis for its formulation was first worked out by physical chemist N. Colin Ba ...
. This means that compounds like benzene, with 4''n'' + 2 π-electrons and aromatic properties in the ground state, become antiaromatic and often adopt less symmetric structures in the excited state.
Aromatic compounds
Importance
Aromatic compounds play key roles in the biochemistry of all living things. The four aromatic amino acidshistidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the d ...
, phenylalanine, tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
, and tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the G ...
each serve as one of the 20 basic building-blocks of proteins. Further, all 5 nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecule ...
s (adenine
Adenine () ( symbol A or Ade) is a nucleobase (a purine derivative). It is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The three others are guanine, cytosine and thymine. Its deri ...
, thymine
Thymine () ( symbol T or Thy) is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidi ...
, cytosine
Cytosine () ( symbol C or Cyt) is one of the four nucleobases found in DNA and RNA, along with adenine, guanine, and thymine (uracil in RNA). It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an ...
, guanine
Guanine () ( symbol G or Gua) is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine (uracil in RNA). In DNA, guanine is paired with cytosine. The guanine nucleoside is c ...
, and uracil
Uracil () (symbol U or Ura) is one of the four nucleobases in the nucleic acid RNA. The others are adenine (A), cytosine (C), and guanine (G). In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced b ...
) that make up the sequence of the genetic code in DNA and RNA are aromatic purines
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines a ...
or pyrimidines
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
. The molecule heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consis ...
contains an aromatic system with 22 π-electrons. Chlorophyll also has a similar aromatic system.
Aromatic compounds are important in industry. Key aromatic hydrocarbons of commercial interest are benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
, toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) a ...
, ''ortho''-xylene and ''para''-xylene. About 35 million tonnes are produced worldwide every year. They are extracted from complex mixtures obtained by the refining of oil or by distillation of coal tar, and are used to produce a range of important chemicals and polymers, including styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
, phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
, aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
, polyester and nylon
Nylon is a generic designation for a family of synthetic polymers composed of polyamides ( repeating units linked by amide links).The polyamides may be aliphatic or semi-aromatic.
Nylon is a silk-like thermoplastic, generally made from pe ...
.
Neutral homocyclics
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
, as well as most other annulene
Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds (' mancude'). They have the general formula CnHn (when ''n'' is an even number) or C''n''H''n''+1 (when ''n'' is an odd number). Th ...
s (with the exception of cyclodecapentaene, because it is non-planar) with the formula C4''n''+2H4''n''+2 where ''n'' is a natural number, such as cyclotetradecaheptaene (''n''=3).
Heterocyclics
Inheterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
aromatics (heteroaromatics), one or more of the atoms in the aromatic ring is of an element other than carbon. This can lessen the ring's aromaticity, and thus (as in the case of furan) increase its reactivity. Other examples include pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a ...
, pyrazine
Pyrazine is a heterocyclic aromatic organic compound with the chemical formula C4H4N2. It is a symmetrical molecule with point group D2h. Pyrazine is less basic than pyridine, pyridazine and pyrimidine. It is a ''"deliquescent crystal or wax-li ...
, pyrrole, imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non-a ...
, pyrazole
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 ( ...
, oxazole
Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak ...
, thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its react ...
, and their benzannulated analogs (benzimidazole
Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a colorless solid.
Preparation
Benzimidazole is produced by condensation of o ...
, for example). In all these examples, the number of π-electrons is 6, due to the π-electrons from the double bonds as well as the two electrons from any lone pair that is in the p-orbital that is in the plane of the aromatic π system. For example, in pyridine, the five sp2-hybridized carbons each have a p-orbital that is perpendicular to the plane of the ring, and each of these p-orbitals contains one π-electron. Additionally, the nitrogen atom is also sp2-hybridized and has one electron in a p-orbital, which adds up to 6 p-electrons, thus making pyridine aromatic. The lone pair on the nitrogen is not part of the aromatic π system. Pyrrole and imidazole are both five membered aromatic rings that contain heteroatoms. In pyrrole, each of the four sp2-hybridized carbons contributes one π-electron, and the nitrogen atom is also sp2-hybridized and contributes two π-electrons from its lone pair, which occupies a p-orbital. In imidazole, both nitrogens are sp2-hybridized; the one in the double bond contributes one electron and the one which is not in the double bond and is in a lone pair contributes two electrons to the π system.
Fused aromatics and polycyclics
Polycyclic aromatic hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. ...
s are molecules containing two or more simple aromatic rings
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found as substructures of ...
fused together by sharing two neighboring carbon atoms. Examples are naphthalene, anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is col ...
, and phenanthrene. In fused aromatics, not all carbon–carbon bonds are necessarily equivalent, as the electrons are not delocalized over the entire molecule. The aromaticity of these molecules can be explained using their orbital picture. Like benzene and other monocyclic aromatic molecules, polycyclics have a cyclic conjugated pi system with p-orbital overlap above and below the plane of the ring.
Substituted aromatics
Manychemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s are aromatic rings with other functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s attached. Examples include trinitrotoluene
Trinitrotoluene (), more commonly known as TNT, more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene, is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reage ...
(TNT), acetylsalicylic acid
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat ...
(aspirin), paracetamol, and the nucleotides of DNA.
Aromatic ions
Aromatic molecules need not be neutral molecules. Ions that satisfy Huckel's rule of 4''n'' + 2 π-electrons in a planar, cyclic, conjugated molecule are considered to be aromatic ions. For example, thecyclopentadienyl anion
In chemistry, the cyclopentadienyl anion or cyclopentadienide is an aromatic species with a formula of and abbreviated as Cp−. It is formed from the deprotonation of the molecule cyclopentadiene.
Properties
The cyclopentadienyl anion i ...
and the cycloheptatrienylium cation are both considered to be aromatic ions, and the azulene
Azulene is an organic compound and an isomer of naphthalene. Naphthalene is colourless, whereas azulene is dark blue. Two terpenoids, vetivazulene (4,8-dimethyl-2-isopropylazulene) and guaiazulene (1,4-dimethyl-7-isopropylazulene), that featur ...
molecule can be approximated as a combination of both.
In order to convert the atom from sp3 to sp2, a carbocation, carbanion, or carbon radical must be formed. These leave sp2-hybridized carbons that can partake in the π system of an aromatic molecule. Like neutral aromatic compounds, these compounds are stable and form easily. The cyclopentadienyl anion is formed very easily and thus 1,3-cyclopentadiene is a very acidic hydrocarbon with a p''K''a of 16. Other examples of aromatic ions include the cyclopropenium cation (2 π-electrons) and cyclooctatetraenyl dianion (10 π electrons).
Atypical aromatic compounds
Aromaticity also occurs in compounds that are not carbocyclic or heterocyclic; inorganic six-membered-ring compounds analogous to benzene have been synthesized. For example,borazine
Borazine, also known as borazole, is a non-polar inorganic compound with the chemical formula B3H6N3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. For thi ...
is a six-membered ring composed of alternating boron and nitrogen atoms, each with one hydrogen attached. It has a delocalized π system and undergoes electrophilic substitution reactions appropriate to aromatic rings rather than reactions expected of non-aromatic molecules.
Quite recently, the aromaticity of planar rings occurring in the Zintl phase
In chemistry, a Zintl phase is a product of a reaction between a group 1 (alkali metal) or group 2 ( alkaline earth metal) and main group metal or metalloid (from groups 13, 14, 15, or 16). It is characterized by intermediate metallic/ ionic bond ...
Li12Si7 was experimentally evinced by Li solid-state NMR. Metal aromaticity is believed to exist in certain clusters of aluminium and gallium, specifically Ga32- and Al42-, for example.
Homoaromaticity
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugated system, conjugation is interrupted by a single sp3 orbital hybridisation, hybridized carbon atom. Although this sp3 center disrupts the continuous ov ...
is the state of systems where conjugation is interrupted by a single sp hybridized carbon atom.
Y-aromaticity is used to describe a Y-shaped, planar
Planar is an adjective meaning "relating to a plane (geometry)".
Planar may also refer to:
Science and technology
* Planar (computer graphics), computer graphics pixel information from several bitplanes
* Planar (transmission line technologies), ...
(flat) molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
with resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied Periodic function, periodic force (or a Fourier analysis, Fourier component of it) is equal or close to a natural frequency of the system ...
bonds. The concept was developed to explain the extraordinary stability and high basicity of the guanidinium
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experie ...
cation. Guanidinium is not a ring molecule, and is cross-conjugated rather than a π system of consecutively attached atoms, but is reported to have its six π-electrons delocalized over the whole molecule. The concept is controversial and some authors emphasize different effects. This has also been suggested as the reason that the trimethylenemethane dication
A dication is any cation, of general formula X2+, formed by the removal of two electrons from a neutral species.
Diatomic dications corresponding to stable neutral species (e.g. formed by removal of two electrons from H2) often decay quickly into ...
is more stable than the butadienyl dication.
σ-aromaticity refers to stabilization arising from the delocalization of sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of s ...
s. It is often invoked in cluster chemistry
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term ''microcluster ...
and is closely related to Wade's Rule
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by ...
. Furthermore, in 2021 a σ-aromatic Th3 complex was reported, indicating that the concept of σ-aromaticity remains relevant for orbitals with principle quantum number 6.
Other symmetries
Möbius aromaticity
In organic chemistry, Möbius aromaticity is a special type of aromaticity believed to exist in a number of organic molecules. In terms of molecular orbital theory these compounds have in common a monocyclic array of molecular orbitals in which th ...
occurs when a cyclic system of molecular orbitals, formed from pπ atomic orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
and populated in a closed shell
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
by 4''n'' (''n'' is an integer) electrons, is given a single half-twist to form a Möbius strip. A π system with 4''n'' electrons in a flat (non-twisted) ring would be antiaromatic, and therefore highly unstable, due to the symmetry of the combinations of p atomic orbitals. By twisting the ring, the symmetry of the system changes and becomes allowed (see also Möbius–Hückel concept In chemistry, the Möbius–Hückel treatment is a methodology used to predict whether a reaction is allowed or forbidden. It is often used alone with the Woodward–Hoffmann approach. The description in this article uses the plus-minus sign nota ...
for details). Because the twist can be left-handed
In human biology, handedness is an individual's preferential use of one hand, known as the dominant hand, due to it being stronger, faster or more dextrous. The other hand, comparatively often the weaker, less dextrous or simply less subject ...
or right-handed
In human biology, handedness is an individual's preferential use of one hand, known as the dominant hand, due to it being stronger, faster or more dextrous. The other hand, comparatively often the weaker, less dextrous or simply less subjecti ...
, the resulting Möbius aromatics are ''dissymmetric'' or chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
. But as of 2012, no Möbius aromatic molecules had been synthesized. Aromatics with two half-twists corresponding to the paradromic topologies were first suggested by Johann Listing. In one form of carbo-benzene, the ring is expanded and contains alkyne and allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon centres (). Allenes are classified as cumulated dienes. The parent compound of this class is propadiene, which ...
groups.
Spherical aromaticity In organic chemistry, spherical aromaticity is formally used to describe an unusually stable nature of some spherical compounds such as fullerenes, polyhedral boranes.
In 2000, Andreas Hirsch and coworkers in Erlangen, Germany, formulated a rule to ...
is aromaticity that occurs in fullerenes. In 2000, Andreas Hirsch and coworkers in Erlangen
Erlangen (; East Franconian: ''Erlang'', Bavarian: ''Erlanga'') is a Middle Franconian city in Bavaria, Germany. It is the seat of the administrative district Erlangen-Höchstadt (former administrative district Erlangen), and with 116,062 inhab ...
, Germany
Germany,, officially the Federal Republic of Germany, is a country in Central Europe. It is the second most populous country in Europe after Russia, and the most populous member state of the European Union. Germany is situated betwe ...
, formulated a rule to determine when a fullerene
A fullerene is an allotrope of carbon whose molecule consists of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecule may be a hollow sphere, ...
would be aromatic. They found that if there were 2(''n'' + 1)2 π-electrons
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
, then the fullerene would display aromatic properties. This follows from the fact that an aromatic fullerene must have full icosahedral
In geometry, an icosahedron ( or ) is a polyhedron with 20 faces. The name comes and . The plural can be either "icosahedra" () or "icosahedrons".
There are infinitely many non- similar shapes of icosahedra, some of them being more symmetrica ...
(or other appropriate) symmetry, so the molecular orbitals must be entirely filled. This is possible only if there are exactly 2(''n'' + 1)2 electrons, where ''n'' is a nonnegative integer.
See also
*Aromatization
Aromatization is a chemical reaction in which an aromatic system is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cyclohexane int ...
* Aromatic amine
In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consi ...
* List of benzo compounds
* Pi interaction In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system c ...
* Avoided crossing
In quantum physics and quantum chemistry, an avoided crossing (sometimes called intended crossing, ''non-crossing'' or anticrossing) is the phenomenon where two eigenvalues of an Hermitian matrix representing a quantum observable and depending on ...
References
External links
* {{Authority control Physical organic chemistry