antibond on:
[Wikipedia]
[Google]
[Amazon]
In
 A molecular orbital becomes antibonding when there is less
A molecular orbital becomes antibonding when there is less
 In molecules with several atoms, some orbitals may be delocalized over more than two atoms. A particular molecular orbital may be ''bonding with respect to some adjacent pairs of atoms'' and ''antibonding with respect to other pairs''. If the bonding interactions outnumber the antibonding interactions, the MO is said to be ''bonding'', whereas, if the antibonding interactions outnumber the bonding interactions, the molecular orbital is said to be ''antibonding''.
For example,
In molecules with several atoms, some orbitals may be delocalized over more than two atoms. A particular molecular orbital may be ''bonding with respect to some adjacent pairs of atoms'' and ''antibonding with respect to other pairs''. If the bonding interactions outnumber the antibonding interactions, the MO is said to be ''bonding'', whereas, if the antibonding interactions outnumber the bonding interactions, the molecular orbital is said to be ''antibonding''.
For example,
chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
ing theory, an antibonding orbital is a type of molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
that weakens the chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing o ...
between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more nodes in the bonding region between the nuclei. The density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematica ...
of the electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
s in the orbital is concentrated outside the bonding region and acts to pull one nucleus away from the other and tends to cause mutual repulsion between the two atoms. This is in contrast to a bonding molecular orbital
In theoretical chemistry, the bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms in a molecule. In MO theory, electrons are portrayed to move in wave ...
, which has a lower energy than that of the separate atoms, and is responsible for chemical bonds.
Diatomic molecules
Antibondingmolecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s (MOs) are normally ''higher'' in energy than bonding molecular orbitals. Bonding and antibonding orbitals form when atoms combine into molecules. If two hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxi ...
atoms are initially far apart, they have identical atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any ...
s. However, as the spacing between the two atoms becomes smaller, the electron wave function
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements m ...
s begin to overlap. The Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulated ...
prohibits any two electrons (e-) in a molecule from having the same set of quantum number
In quantum physics and chemistry, quantum numbers describe values of conserved quantities in the dynamics of a quantum system. Quantum numbers correspond to eigenvalues of operators that commute with the Hamiltonian—quantities that can be ...
s. Therefore each original atomic orbital of the isolated atoms (for example, the ground state energy level, 1''s'') splits into two molecular orbitals belonging to the pair, one lower in energy than the original atomic level and one higher. The orbital which is in a lower energy state than the orbitals of the separate atoms is the bonding orbital, which is more stable and promotes the bonding of the two H atoms into H2. The higher-energy orbital is the antibonding orbital, which is less stable and opposes bonding if it is occupied. In a molecule such as H2, the two electrons normally occupy the lower-energy bonding orbital, so that the molecule is more stable than the separate H atoms.
 A molecular orbital becomes antibonding when there is less
A molecular orbital becomes antibonding when there is less electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
between the two nuclei than there would be if there were no bonding interaction at all. When a molecular orbital changes sign (from positive to negative) at a ''nodal plane'' between two atoms, it is said to be ''antibonding with respect to those atoms''. Antibonding orbitals are often labelled with an asterisk
The asterisk ( ), from Late Latin , from Ancient Greek , ''asteriskos'', "little star", is a typographical symbol. It is so called because it resembles a conventional image of a heraldic star.
Computer scientists and mathematicians often vo ...
(*) on molecular orbital diagrams.
In homonuclear
Homonuclear molecules, or homonuclear species, are molecules composed of only one element. Homonuclear molecules may consist of various numbers of atoms. The size of the molecule an element can form depends on the element's properties, and some el ...
diatomic molecules, σ* (''sigma star'') antibonding orbitals have no nodal planes passing through the two nuclei, like sigma bonds
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of ...
, and π* (''pi star'') orbitals have one nodal plane passing through the two nuclei, like pi bonds
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbita ...
. The Pauli exclusion principle
In quantum mechanics, the Pauli exclusion principle states that two or more identical particles with half-integer spins (i.e. fermions) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulated ...
dictates that no two electrons in an interacting system may have the same quantum state. If the bonding orbitals are filled, then any additional electrons will occupy antibonding orbitals. This occurs in the He2 molecule, in which both the 1sσ and 1sσ* orbitals are filled. Since the ''antibonding orbital is more antibonding than the bonding orbital is bonding'', the molecule has a higher energy than two separated helium atoms, and it is therefore unstable.
Polyatomic molecules
 In molecules with several atoms, some orbitals may be delocalized over more than two atoms. A particular molecular orbital may be ''bonding with respect to some adjacent pairs of atoms'' and ''antibonding with respect to other pairs''. If the bonding interactions outnumber the antibonding interactions, the MO is said to be ''bonding'', whereas, if the antibonding interactions outnumber the bonding interactions, the molecular orbital is said to be ''antibonding''.
For example,
In molecules with several atoms, some orbitals may be delocalized over more than two atoms. A particular molecular orbital may be ''bonding with respect to some adjacent pairs of atoms'' and ''antibonding with respect to other pairs''. If the bonding interactions outnumber the antibonding interactions, the MO is said to be ''bonding'', whereas, if the antibonding interactions outnumber the bonding interactions, the molecular orbital is said to be ''antibonding''.
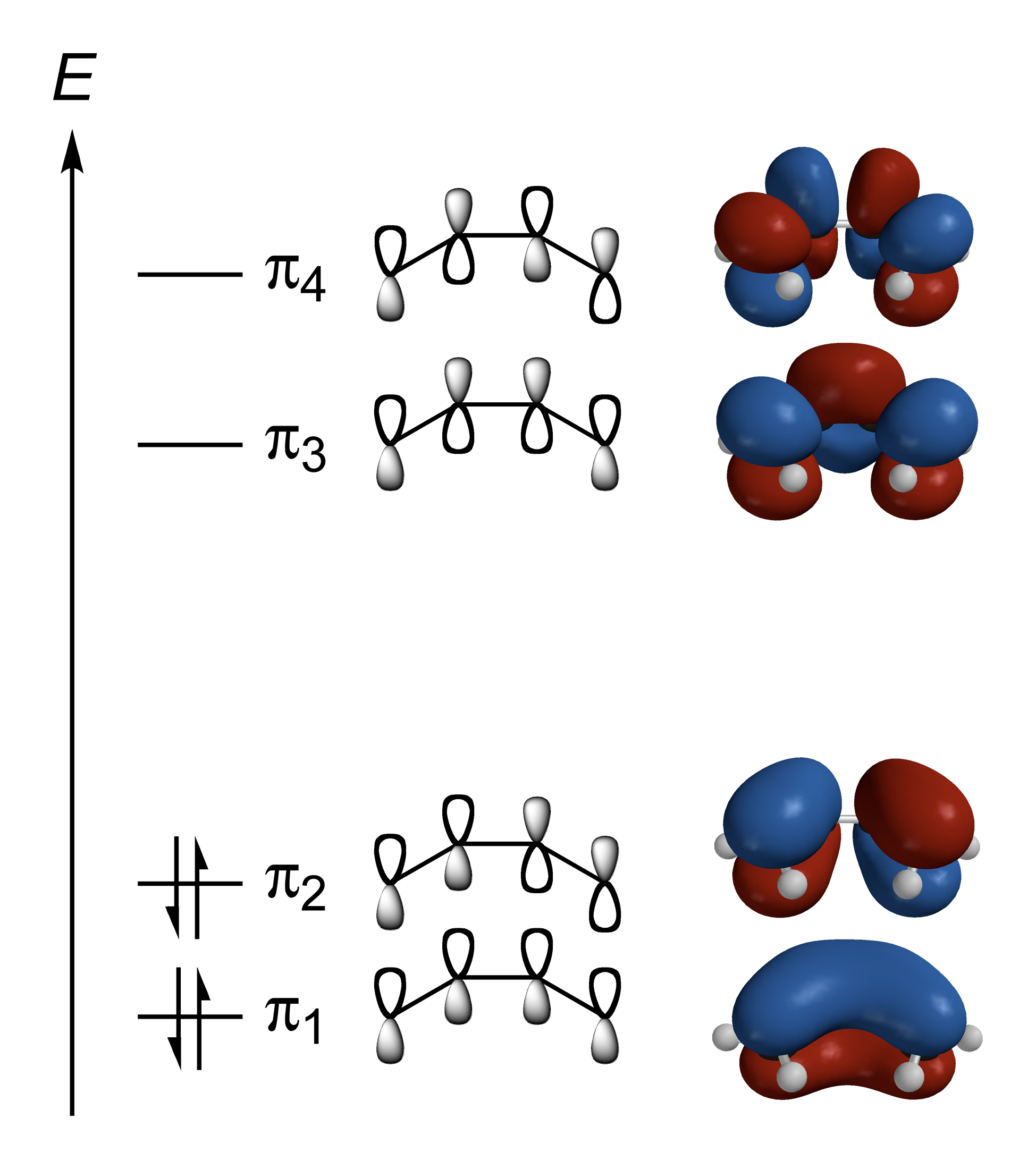
For example, butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
has pi orbitals which are delocalized over all four carbon atoms. There are two bonding pi orbitals which are occupied in the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
: π1 is bonding between all carbons, while π2 is bonding between C1 and C2 and between C3 and C4, and antibonding between C2 and C3. There are also antibonding pi orbitals with two and three antibonding interactions as shown in the diagram; these are vacant in the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
, but may be occupied in excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers ...
s.
Similarly benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen a ...
with six carbon atoms has three bonding pi orbitals and three antibonding pi orbitals.
Since each carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes u ...
atom contributes one electron to the π-system of benzene, there are six pi electrons which fill the three lowest-energy pi molecular orbitals (the bonding pi orbitals).
Antibonding orbitals are also important for explaining chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breakin ...
s in terms of molecular orbital theory. Roald Hoffmann
Roald Hoffmann (born Roald Safran; July 18, 1937) is a Polish-American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He has also published plays and poetry. He is the Frank H. T. Rhodes Professor of Humane Letters, Emeritus, at ...
and Kenichi Fukui shared the 1981 Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
for their work and further development of qualitative molecular orbital explanations for chemical reactions.
See also
*Bonding molecular orbital
In theoretical chemistry, the bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms in a molecule. In MO theory, electrons are portrayed to move in wave ...
*Valence and conduction bands
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in w ...
*Valence bond theory
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of ...
*Molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
*Conjugated system
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented ...
References
Further reading
* Orchin, M. Jaffe, H.H. (1967) ''The Importance of Antibonding Orbitals''. Houghton Mifflin. ISBN B0006BPT5O {{Chemical bonding theory Chemical bonding