alkali metal oxide on:
[Wikipedia]
[Google]
[Amazon]
 The
The
 * Hexarubidium monoxide (Rb6O)
* Nonarubidium dioxide (Rb9O2)
* Tricaesium monoxide (Cs3O) is a dark green solid.
* Tetracaesium monoxide (Cs4O)
* Heptacaesium monoxide (Cs7O)
* Tricaesium dioxide (Cs3O2)
* Heptacaesium dioxide (Cs7O2)
* Undecacaesium trioxide (Cs11O3)
* Undecacaesium monorubidium trioxide (Cs11RbO3)
* Undecacaesium dirubidium trioxide (Cs11Rb2O3)
* Undecacaesium trirubidium trioxide (Cs11Rb3O3)
* Hexarubidium monoxide (Rb6O)
* Nonarubidium dioxide (Rb9O2)
* Tricaesium monoxide (Cs3O) is a dark green solid.
* Tetracaesium monoxide (Cs4O)
* Heptacaesium monoxide (Cs7O)
* Tricaesium dioxide (Cs3O2)
* Heptacaesium dioxide (Cs7O2)
* Undecacaesium trioxide (Cs11O3)
* Undecacaesium monorubidium trioxide (Cs11RbO3)
* Undecacaesium dirubidium trioxide (Cs11Rb2O3)
* Undecacaesium trirubidium trioxide (Cs11Rb3O3)
 *
*
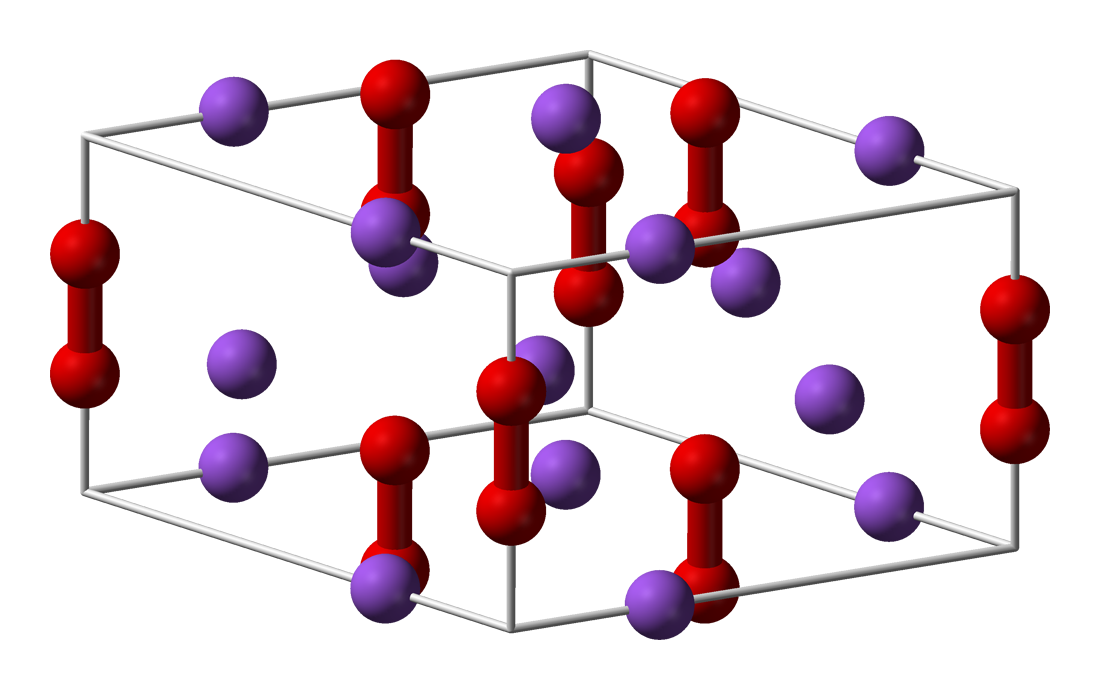 * Lithium peroxide (Li2O2) is a white solid that melts at 195 °C. It reacts with
* Lithium peroxide (Li2O2) is a white solid that melts at 195 °C. It reacts with
 * Lithium superoxide (LiO2) has only been isolated in matrix isolation at 15 K.
*
* Lithium superoxide (LiO2) has only been isolated in matrix isolation at 15 K.
*
 * Lithium ozonide (LiO3) is a red solid which is produced from caesium ozonide via an ion-exchange process.
*
* Lithium ozonide (LiO3) is a red solid which is produced from caesium ozonide via an ion-exchange process.
*
 The
The alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s react with oxygen to form several different compounds: suboxides, oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s, peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
s, sesquioxide
A sesquioxide is an oxide of an element (or radical), where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide and phosphorus(III) oxide are sesquioxides.
Many sesquioxid ...
s, superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of t ...
s, and ozonide
Ozonide is the polyatomic anion . Cyclic organic compounds formed by the addition of ozone () to an alkene are also called ozonides.
Ionic ozonides
Inorganic ozonides are dark red salts. The anion has the bent shape of the ozone molecule.
In ...
s. They all react violently with water.
Alkali metal suboxides
 * Hexarubidium monoxide (Rb6O)
* Nonarubidium dioxide (Rb9O2)
* Tricaesium monoxide (Cs3O) is a dark green solid.
* Tetracaesium monoxide (Cs4O)
* Heptacaesium monoxide (Cs7O)
* Tricaesium dioxide (Cs3O2)
* Heptacaesium dioxide (Cs7O2)
* Undecacaesium trioxide (Cs11O3)
* Undecacaesium monorubidium trioxide (Cs11RbO3)
* Undecacaesium dirubidium trioxide (Cs11Rb2O3)
* Undecacaesium trirubidium trioxide (Cs11Rb3O3)
* Hexarubidium monoxide (Rb6O)
* Nonarubidium dioxide (Rb9O2)
* Tricaesium monoxide (Cs3O) is a dark green solid.
* Tetracaesium monoxide (Cs4O)
* Heptacaesium monoxide (Cs7O)
* Tricaesium dioxide (Cs3O2)
* Heptacaesium dioxide (Cs7O2)
* Undecacaesium trioxide (Cs11O3)
* Undecacaesium monorubidium trioxide (Cs11RbO3)
* Undecacaesium dirubidium trioxide (Cs11Rb2O3)
* Undecacaesium trirubidium trioxide (Cs11Rb3O3)
Alkali metal oxides
 *
*Lithium oxide
Lithium oxide (Lithium, Oxygen, O) or lithia is an Inorganic compound, inorganic chemical compound. It is a white or pale yellow solid. Although not specifically important, many materials are assessed on the basis of their Li2O content. For examp ...
(Li2O) is the lightest alkali metal oxide and a white solid. It melts at 1570 °C.
*Sodium oxide
Sodium oxide is a chemical compound with the formula . It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and f ...
(Na2O) is a white solid that melts at 1132 °C and decomposes at 1950 °C. It is a component of glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window pane ...
.
*Potassium oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertil ...
(K2O) is a pale yellow solid that decomposes at 350 °C.
*Rubidium oxide
Rubidium oxide is the chemical compound with the formula . Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. The rubidium content in minerals is often calculated and quoted in terms of . ...
(Rb2O) is a yellow solid that melts at 500 °C.
* Caesium oxide (Cs2O) is a yellow-orange solid that melts at 490 °C.
Alkali metal peroxides
 * Lithium peroxide (Li2O2) is a white solid that melts at 195 °C. It reacts with
* Lithium peroxide (Li2O2) is a white solid that melts at 195 °C. It reacts with carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
to form lithium carbonate
Lithium carbonate is an inorganic compound, the lithium salt of carbonic acid with the chemical formula, formula . This white Salt (chemistry), salt is widely used in processing metal oxides. It is on the WHO Model List of Essential Medicines, Wor ...
and oxygen.
*Sodium peroxide
Sodium peroxide is an inorganic compound with the formula Na2 O2. This yellowish solid is the product of sodium ignited in excess oxygen. It is a strong base. This metal peroxide exists in several hydrates and peroxyhydrates including Na2O2·2H2 ...
(Na2O2) is a pale yellow solid that melts at 460 °C and decomposes at 657 °C.
*Potassium peroxide
Potassium peroxide is an inorganic compound with the molecular formula K2O2. It is formed as potassium reacts with oxygen in the air, along with potassium oxide (K2O) and potassium superoxide (KO2).
Potassium peroxide reacts with water to for ...
(K2O2) is a yellow solid that melts at 490 °C.
* Rubidium peroxide (Rb2O2) is produced when rubidium stands in air.
* Caesium peroxide (Cs2O2) is produced by the decomposition of caesium oxide above 400 °C.
Alkali metal sesquioxides
*Rubidium sesquioxide
Rubidium sesquioxide is a chemical compound with the formula or more accurately . In terms of oxidation states, Rubidium in this compound has a nominal charge of +1, and the oxygen is a mixed peroxide () and superoxide () for a structural formula ...
(Rb4O6) is a black solid.
* Caesium sesquioxide (Cs4O6) is a black solid.
Alkali metal superoxides
 * Lithium superoxide (LiO2) has only been isolated in matrix isolation at 15 K.
*
* Lithium superoxide (LiO2) has only been isolated in matrix isolation at 15 K.
*Sodium superoxide
Sodium superoxide is the inorganic compound with the formula Na O2. This yellow-orange solid is a salt of the superoxide anion. It is an intermediate in the oxidation of sodium by oxygen.
Preparation
NaO2 is prepared by treating sodium peroxid ...
(NaO2) is a yellow-orange solid that melts at 551.7 °C. It is made by the high-pressure oxidation of sodium peroxide.
*Potassium superoxide
Potassium superoxide is an inorganic compound with the formula . It is a yellow paramagnetic solid that decomposes in moist air. It is a rare example of a stable salt of the superoxide anion. It is used as a scrubber, dehumidifier, and gene ...
(KO2) is a yellow solid that decomposes at 560 °C. It is used as a CO2 scrubber, H2O dehumidifier, and O2 generator in rebreather
A rebreather is a breathing apparatus that absorbs the carbon dioxide of a user's exhaled breath to permit the rebreathing (recycling) of the substantial unused oxygen content, and unused inert content when present, of each breath. Oxygen is a ...
s, spacecraft
A spacecraft is a vehicle that is designed spaceflight, to fly and operate in outer space. Spacecraft are used for a variety of purposes, including Telecommunications, communications, Earth observation satellite, Earth observation, Weather s ...
, submarines
A submarine (often shortened to sub) is a watercraft capable of independent operation underwater. (It differs from a submersible, which has more limited underwater capability.) The term "submarine" is also sometimes used historically or info ...
, and spacesuit
A space suit (or spacesuit) is an environmental suit used for protection from the harsh Space environment, environment of outer space, mainly from its Vacuum (outer space), vacuum as a highly specialized pressure suit, but also its temperatu ...
life support system
A life-support system is the combination of equipment that allows survival in an environment or situation that would not support that life in its absence. It is generally applied to systems supporting human life in situations where the outside ...
s.
*Rubidium superoxide
Rubidium superoxide or rubidium hyperoxide is a chemical compound with the chemical formula . In terms of oxidation states, the negatively charged superoxide and positively charged rubidium give it a structural formula of .
Chemistry
It can be cre ...
(RbO2) is produced when rubidium burns in air.
*Caesium superoxide
Caesium superoxide is a chemical compound with the chemical formula . It consists of caesium cations and superoxide anions . It is an orange solid.
Preparation
Burning caesium in excess oxygen will produce caesium superoxide.
:
Properties
...
(CsO2) is produced when caesium burns in air.
Alkali metal ozonides
 * Lithium ozonide (LiO3) is a red solid which is produced from caesium ozonide via an ion-exchange process.
*
* Lithium ozonide (LiO3) is a red solid which is produced from caesium ozonide via an ion-exchange process.
*Sodium ozonide
Sodium ozonide (NaO3) is an oxygen-rich compound of sodium. As an ozonide, it contains the ozonide anion ().
Some experiments report creating sodium ozonide by applying ozone to sodium hydroxide, but the substance was not pure, and the claimed st ...
(NaO3) is a red solid which is produced from caesium ozonide via an ion-exchange process.
*Potassium ozonide
Potassium ozonide is an oxygen rich compound of potassium. It is an ozonide, meaning it contains the ozonide anion (O3−). In polarized light, it shows pleochroism. Hybrid functional calculations have predicted the compound is an insulator with ...
(KO3) is a dark red solid which is produced when potassium is burned in ozone or exposed to air for years.
*Rubidium ozonide
Rubidium ozonide is an oxygen rich compound of rubidium. It is an ozonide, meaning it contains the ozonide anion (O3−).
It can be created by reacting rubidium superoxide (RbO2) with ozone (O3) in a liquid ammonia solution.
:
The chemical form ...
(RbO3) is a dark red solid which is produced when rubidium is burned in ozone.
*Caesium ozonide
Caesium ozonide is an oxygen-rich chemical compound of caesium, with the chemical formula . It consists of caesium cations and ozonide anions . It can be formed by reacting ozone with caesium superoxide:
:
The compound reacts strongly with any ...
(CsO3) is a dark red solid which is produced when caesium is burned in ozone. F. A. Cotton and G. Wilkinson "Advanced Inorganic Chemistry", 5th edition (1988), p.462
References
{{reflist Alkali metals Oxides