Aldol reaction on:
[Wikipedia]
[Google]
[Amazon]
The aldol reaction is a means of forming carbon–carbon bonds in  A typical modern aldol
A typical modern aldol 

 Acid-catalyzed dehydration
Acid-catalyzed dehydration

 Base-catalyzed dehydration (frequently written incorrectly as a single step, see E1cB elimination reaction)
Base-catalyzed dehydration (frequently written incorrectly as a single step, see E1cB elimination reaction)
 Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a
Although only a catalytic amount of base is required in some cases, the more usual procedure is to use a

 In this reaction, two unsymmetrical ketones are being condensed using sodium ethoxide. The basicity of sodium ethoxide is such that it cannot fully deprotonate either of the ketones, but can produce small amounts of the sodium enolate of both ketones. This means that, in addition to being potential aldol electrophiles, both ketones may also act as nucleophiles via their sodium enolate. Two electrophiles and two nucleophiles, then, have potential to result in four possible products:
In this reaction, two unsymmetrical ketones are being condensed using sodium ethoxide. The basicity of sodium ethoxide is such that it cannot fully deprotonate either of the ketones, but can produce small amounts of the sodium enolate of both ketones. This means that, in addition to being potential aldol electrophiles, both ketones may also act as nucleophiles via their sodium enolate. Two electrophiles and two nucleophiles, then, have potential to result in four possible products:
 Thus, if one wishes to obtain only one of the cross-products, one must control which carbonyl becomes the nucleophilic enol/enolate and which remains in its electrophilic carbonyl form.
Thus, if one wishes to obtain only one of the cross-products, one must control which carbonyl becomes the nucleophilic enol/enolate and which remains in its electrophilic carbonyl form.
 The malonate is particularly easy to deprotonate because the α position is flanked by more than one carbonyl. Double-activation makes the enolate more stable, so not as strong a base is required to form it. An extension of this effect can allow control over which of the two carbonyl reactants becomes the enolate even if both do have α hydrogens. If one partner is considerably more acidic than the other, the most acidic proton is abstracted by the base and an enolate is formed at that carbonyl while the carbonyl that is less acidic is not affected by the base. This type of control works only if the difference in acidity is large enough and no excess of base is used for the reaction. A typical substrate for this situation is when the deprotonatable position is activated by more than one carbonyl-like group. Common examples include a CH2 group flanked by two carbonyls or nitriles (see for example the Knoevenagel condensation and the first steps of the
The malonate is particularly easy to deprotonate because the α position is flanked by more than one carbonyl. Double-activation makes the enolate more stable, so not as strong a base is required to form it. An extension of this effect can allow control over which of the two carbonyl reactants becomes the enolate even if both do have α hydrogens. If one partner is considerably more acidic than the other, the most acidic proton is abstracted by the base and an enolate is formed at that carbonyl while the carbonyl that is less acidic is not affected by the base. This type of control works only if the difference in acidity is large enough and no excess of base is used for the reaction. A typical substrate for this situation is when the deprotonatable position is activated by more than one carbonyl-like group. Common examples include a CH2 group flanked by two carbonyls or nitriles (see for example the Knoevenagel condensation and the first steps of the
(2SR,3RS)-2,4-Dimethyl-3-Hydroxypentanoic Acid
, '' Org. Synth.'', Coll. Vol. 7, p.185 (1990); Vol. 63, p.89 (1985). Kinetic control means that the forward aldol addition reaction must be significantly faster than the reverse retro-aldol reaction. For this approach to succeed, two other conditions must also be satisfied; it must be possible to quantitatively form the enolate of one partner, and the forward aldol reaction must be significantly faster than the transfer of the enolate from one partner to another. Common kinetic control conditions involve the formation of the enolate of a ketone with LDA at −78 °C, followed by the slow addition of an aldehyde.
 For
For 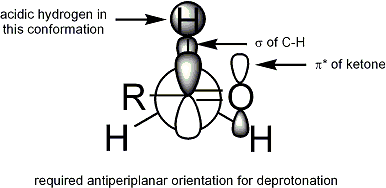
 For ketones, most enolization conditions give ''Z'' enolates. For
For ketones, most enolization conditions give ''Z'' enolates. For  The stereoselective formation of enolates has been rationalized with the Ireland model, although its validity is somewhat questionable. In most cases, it is not known which, if any, intermediates are
The stereoselective formation of enolates has been rationalized with the Ireland model, although its validity is somewhat questionable. In most cases, it is not known which, if any, intermediates are
 The trisubstituted enolate is considered the kinetic enolate, while the tetrasubstituted enolate is considered the thermodynamic enolate. The alpha hydrogen deprotonated to form the kinetic enolate is less hindered, and therefore deprotonated more quickly. In general, tetrasubstituted olefins are more stable than trisubstituted olefins due to hyperconjugative stabilization. The ratio of enolate regioisomers is heavily influenced by the choice of base. For the above example, kinetic control may be established with LDA at −78 °C, giving 99:1 selectivity of kinetic: thermodynamic enolate, while thermodynamic control may be established with triphenylmethyllithium at room temperature, giving 10:90 selectivity.
In general, kinetic enolates are favored by cold temperatures, conditions that give relatively ionic metal–oxygen bonding, and rapid deprotonation using a slight excess of a strong, sterically hindered base. The large base only deprotonates the more accessible hydrogen, and the low temperatures and excess base help avoid equilibration to the more stable alternate enolate after initial enolate formation. Thermodynamic enolates are favored by longer equilibration times at higher temperatures, conditions that give relatively covalent metal–oxygen bonding, and use of a slight sub-stoichiometric amount of strong base. By using insufficient base to deprotonate all of the carbonyl molecules, the enolates and carbonyls can exchange protons with each other and equilibrate to their more stable isomer. Using various metals and solvents can provide control over the amount of ionic character in the metal–oxygen bond.
The trisubstituted enolate is considered the kinetic enolate, while the tetrasubstituted enolate is considered the thermodynamic enolate. The alpha hydrogen deprotonated to form the kinetic enolate is less hindered, and therefore deprotonated more quickly. In general, tetrasubstituted olefins are more stable than trisubstituted olefins due to hyperconjugative stabilization. The ratio of enolate regioisomers is heavily influenced by the choice of base. For the above example, kinetic control may be established with LDA at −78 °C, giving 99:1 selectivity of kinetic: thermodynamic enolate, while thermodynamic control may be established with triphenylmethyllithium at room temperature, giving 10:90 selectivity.
In general, kinetic enolates are favored by cold temperatures, conditions that give relatively ionic metal–oxygen bonding, and rapid deprotonation using a slight excess of a strong, sterically hindered base. The large base only deprotonates the more accessible hydrogen, and the low temperatures and excess base help avoid equilibration to the more stable alternate enolate after initial enolate formation. Thermodynamic enolates are favored by longer equilibration times at higher temperatures, conditions that give relatively covalent metal–oxygen bonding, and use of a slight sub-stoichiometric amount of strong base. By using insufficient base to deprotonate all of the carbonyl molecules, the enolates and carbonyls can exchange protons with each other and equilibrate to their more stable isomer. Using various metals and solvents can provide control over the amount of ionic character in the metal–oxygen bond.
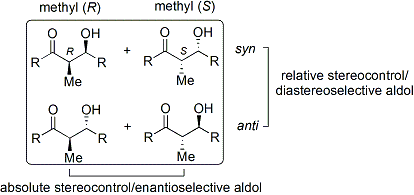
 The convention applies when propionate (or higher order) nucleophiles are added to aldehydes. The ''R'' group of the ketone and the ''R group of the aldehyde are aligned in a "zig zag" pattern in the plane of the paper (or screen), and the disposition of the formed stereocenters is deemed ''syn'' or ''anti'', depending if they are on the same or opposite sides of the main chain.
Older papers use the '' erythro/threo'' nomenclature familiar from saccharide chemistry.
The convention applies when propionate (or higher order) nucleophiles are added to aldehydes. The ''R'' group of the ketone and the ''R group of the aldehyde are aligned in a "zig zag" pattern in the plane of the paper (or screen), and the disposition of the formed stereocenters is deemed ''syn'' or ''anti'', depending if they are on the same or opposite sides of the main chain.
Older papers use the '' erythro/threo'' nomenclature familiar from saccharide chemistry.


 For example, boron–carbon and boron–oxygen bonds are 1.4–1.5 Å and 1.5–1.6 Å in length, respectively, whereas typical metal-carbon and metal-oxygen bonds are 1.9–2.2 Å and 2.0–2.2 Å in length, respectively. The use of boron rather than a metal "tightens" the
For example, boron–carbon and boron–oxygen bonds are 1.4–1.5 Å and 1.5–1.6 Å in length, respectively, whereas typical metal-carbon and metal-oxygen bonds are 1.9–2.2 Å and 2.0–2.2 Å in length, respectively. The use of boron rather than a metal "tightens" the
 In the case of an E enolate, the dominant control element is allylic 1,3-strain whereas in the case of a Z enolate, the dominant control element is the avoidance of 1,3-diaxial interactions. The general model is presented below:
In the case of an E enolate, the dominant control element is allylic 1,3-strain whereas in the case of a Z enolate, the dominant control element is the avoidance of 1,3-diaxial interactions. The general model is presented below:
 For clarity, the stereocenter on the enolate has been
For clarity, the stereocenter on the enolate has been 
 Since ''Z'' enolates must react through a
Since ''Z'' enolates must react through a 
Diastereoselective Aldol Condensation Using A Chiral Oxazolidinone Auxiliary: (2S*,3S*)-3-Hydroxy-3-Phenyl-2-Methylpropanoic Acid
,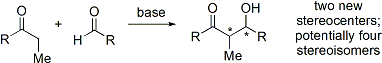
 In the case of the Evans' method, the chiral auxiliary appended is an
In the case of the Evans' method, the chiral auxiliary appended is an 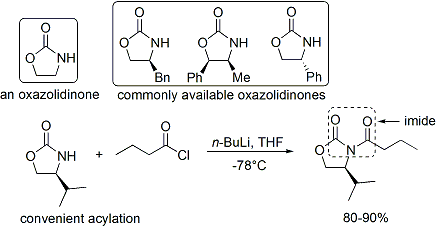 The
The  Often, a single
Often, a single  Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective: and anti selective:
Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective: and anti selective:
 In the syn-selective reactions, both enolization methods give the ''Z'' enolate, as expected; however, the stereochemical outcome of the reaction is controlled by the methyl stereocenter, rather than the chirality of the oxazolidinone. The methods described allow the stereoselective assembly of
In the syn-selective reactions, both enolization methods give the ''Z'' enolate, as expected; however, the stereochemical outcome of the reaction is controlled by the methyl stereocenter, rather than the chirality of the oxazolidinone. The methods described allow the stereoselective assembly of
 Intramolecular aldol reaction is the condensation reaction of two
Intramolecular aldol reaction is the condensation reaction of two  Intramolecular aldol reactions have been widely used in total syntheses of various natural products, especially alkaloids and steroids. An example is the application of an intramolecular aldol reaction in the ring closure step for total synthesis of (+)- Wortmannin by Shigehisa, et al. (Figure 2).
Intramolecular aldol reactions have been widely used in total syntheses of various natural products, especially alkaloids and steroids. An example is the application of an intramolecular aldol reaction in the ring closure step for total synthesis of (+)- Wortmannin by Shigehisa, et al. (Figure 2).
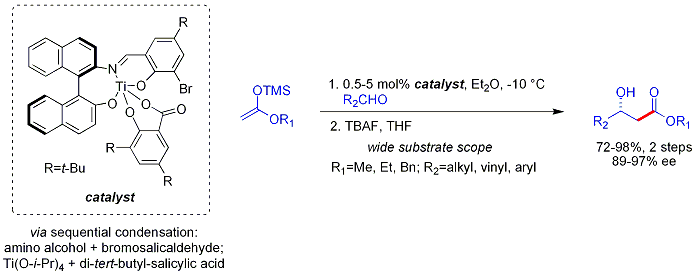 The analogous
The analogous 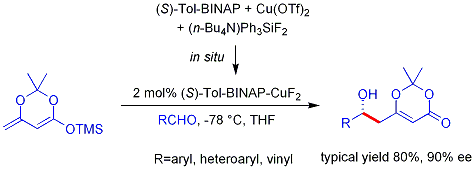

 This reaction is known as the Hajos-Parrish reaction (also known as the Hajos-Parrish-Eder-Sauer-Wiechert reaction, referring to a contemporaneous report from Schering of the reaction under harsher conditions). Under the Hajos-Parrish conditions only a catalytic amount of proline is necessary (3 mol%). There is no danger of an achiral background reaction because the transient enamine intermediates are much more nucleophilic than their parent ketone enols. This strategy offers a simple way of generating enantioselectivity in reactions without using transition metals, which have the possible disadvantages of being toxic or expensive.
Proline-catalyzed aldol reactions do not show any non-linear effects (the enantioselectivity of the products is directly proportional to the enantiopurity of the catalyst). Combined with
This reaction is known as the Hajos-Parrish reaction (also known as the Hajos-Parrish-Eder-Sauer-Wiechert reaction, referring to a contemporaneous report from Schering of the reaction under harsher conditions). Under the Hajos-Parrish conditions only a catalytic amount of proline is necessary (3 mol%). There is no danger of an achiral background reaction because the transient enamine intermediates are much more nucleophilic than their parent ketone enols. This strategy offers a simple way of generating enantioselectivity in reactions without using transition metals, which have the possible disadvantages of being toxic or expensive.
Proline-catalyzed aldol reactions do not show any non-linear effects (the enantioselectivity of the products is directly proportional to the enantiopurity of the catalyst). Combined with 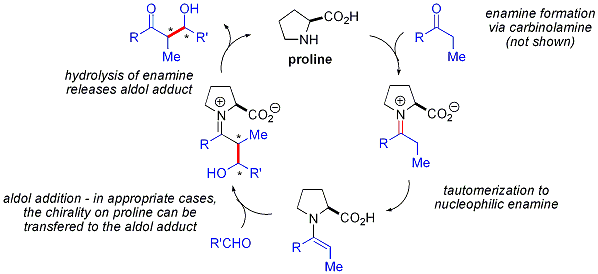 This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can
This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can 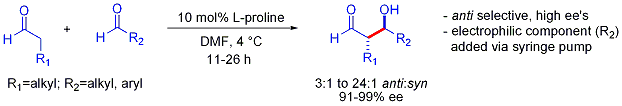 In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected
In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected 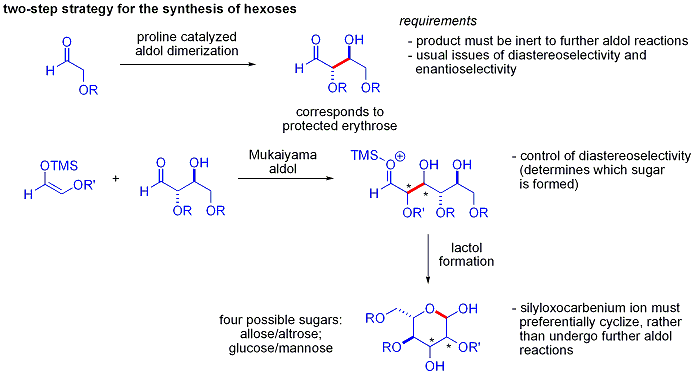 The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha- silyloxy substituents were suitable for this reaction, while aldehydes bearing
The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha- silyloxy substituents were suitable for this reaction, while aldehydes bearing 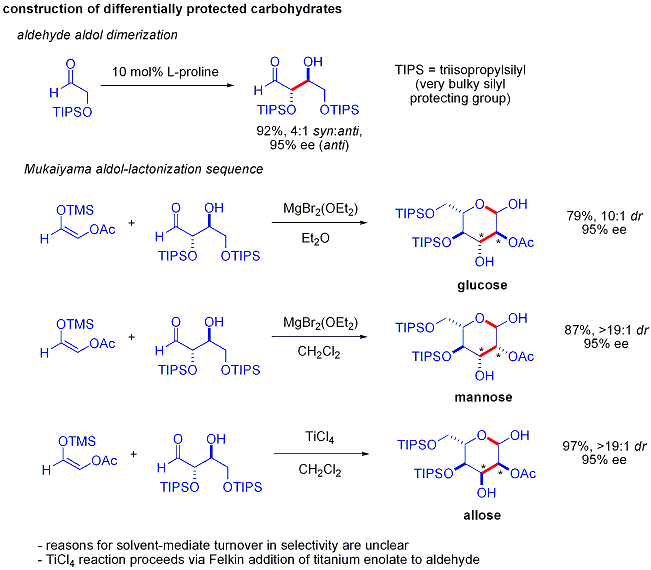
 One approach, demonstrated by Evans, is to silylate the aldol adduct. A silicon reagent such as
One approach, demonstrated by Evans, is to silylate the aldol adduct. A silicon reagent such as 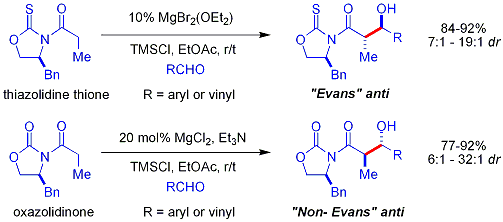
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
.
Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
compounds (the original experiments used aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s) to form a new β-hydroxy carbonyl compound. These products are known as '' aldols'', from the ''ald''ehyde + alcoh''ol'', a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic.
For example, the aldol reaction has been used in the large-scale production of the commodity chemical pentaerythritol
Pentaerythritol is an organic compound with the formula C(CH2OH)4. Classified as a polyol, it is a white solid. Pentaerythritol is a building block for the synthesis and production of explosives, plastics, paints, appliances, cosmetics, and many o ...
and the synthesis of the heart disease drug Lipitor (atorvastatin
Atorvastatin is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a first-line treatment. It is taken by mouth.
Common ...
, calcium salt).
The aldol reaction unites two relatively simple molecules into a more complex one. Increased complexity arises because up to two new stereogenic centers (on the α- and β-carbon of the aldol adduct, marked with asterisks in the scheme below) are formed. Modern methodology is capable of not only allowing aldol reactions to proceed in high yield but also controlling both the relative and absolute configuration
Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity (or group) and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules, where carbon is bonde ...
of these stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups c ...
s. This ability to selectively synthesize a particular stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in ...
is significant because stereoisomers can have distinctive chemical and biological properties.
For example, stereogenic aldol units are especially common in polyketide
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynth ...
s, a class of molecules found in biological organisms. In nature, polyketides are synthesized by enzymes that effect iterative Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Ra ...
s. The 1,3-dicarbonyl products of these reactions can then be variously derivatized to produce a wide variety of interesting structures. Often, such derivitization involves the reduction of one of the carbonyl groups, producing the aldol subunit. Some of these structures have potent biological properties: the immunosuppressant
Immunosuppressive drugs, also known as immunosuppressive agents, immunosuppressants and antirejection medications, are drugs that inhibit or prevent activity of the immune system.
Classification
Immunosuppressive drugs can be classified in ...
FK506
Tacrolimus, sold under the brand name Prograf among others, is an immunosuppressive drug. After allogeneic organ transplant, the risk of organ rejection is moderate. To lower the risk of organ rejection, tacrolimus is given. The drug can also ...
, the anti-tumor agent discodermolide
(+)-Discodermolide is a polyketide natural product found to stabilize microtubules. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge '' Discodermia dissoluta'' i ...
, or the antifungal agent amphotericin B
Amphotericin B is an antifungal medication used for serious mycosis, fungal infections and leishmaniasis. The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candida infections, candidiasis, coccidioidomy ...
, for example. Although the synthesis of many such compounds was once considered nearly impossible, aldol methodology has allowed their efficient synthesis in many cases.
addition reaction
In organic chemistry, an addition reaction is, in simplest terms, an organic reaction where two or more molecules combine to form a larger one (the adduct)..
Addition reactions are limited to chemical compounds that have multiple bonds, such as ...
, shown above, might involve the nucleophilic addition of a ketone enolate to an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
. Once formed, the aldol product can sometimes lose a molecule of water to form an α,β-unsaturated carbonyl compound
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing ...
. This is called ''aldol condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to ...
''. A variety of nucleophiles may be employed in the aldol reaction, including the enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The t ...
s, enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
s, and enol ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be ...
s of ketones, aldehydes, and many other carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
compounds. The electrophilic partner is usually an aldehyde or ketone (many variations, such as the Mannich reaction
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl () functional group by formaldehyde () and a primary or secondary amine () or ammonia (). ...
, exist). When the nucleophile and electrophile are different, the reaction is called a ''crossed aldol reaction''; on the converse, when the nucleophile and electrophile are the same, the reaction is called an ''aldol dimerization''.

Mechanisms
The aldol reaction may proceed by two distinct mechanisms. Carbonyl compounds, such as aldehydes and ketones, can be converted to enols or enol ethers. These species, being nucleophilic at the α-carbon, can attack especially reactive protonated carbonyls such as protonated aldehydes. This is the 'enol mechanism'. Carbonyl compounds, beingcarbon acid
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3CH ...
s, can also be deprotonated to form enolates, which are much more nucleophilic than enols or enol ethers and can attack electrophiles directly. The usual electrophile is an aldehyde, since ketones are much less reactive. This is the 'enolate mechanism'.
Despite the attractiveness of the aldol manifold, there are several problems that need to be addressed to render the process catalytic and effective. The first problem is a thermodynamic one: most aldol reactions are reversible. Furthermore, the equilibrium is also just barely on the side of the products in the case of simple aldehyde–ketone aldol reactions. If the conditions are particularly harsh (e.g.: NaOMe/MeOH/reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions ...
), condensation may occur, but this can usually be avoided with mild reagents and low temperatures (e.g., LDA (a strong base), THF, −78 °C). Although the aldol addition usually proceeds to near completion under irreversible conditions, the isolated aldol adducts are sensitive to base-induced retro-aldol cleavage to return starting materials. In contrast, retro-aldol condensations are rare, but possible. This is the basis of the catalytic strategy of class I aldolases in nature, as well as numerous small-molecule amine catalysts.
Enol mechanism
When an acid catalyst is used, the initial step in the reaction mechanism involves acid-catalyzed tautomerization of the carbonyl compound to the enol. The acid also serves to activate the carbonyl group of ''another molecule'' by protonation, rendering it highly electrophilic. The enol is nucleophilic at the α-carbon, allowing it to attack the protonated carbonyl compound, leading to the aldol afterdeprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
. This usually dehydrates to give the unsaturated carbonyl compound. The scheme shows a typical acid-catalyzed self-condensation of an aldehyde.
Acid-catalyzed aldol mechanism
Enolate mechanism
If thecatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
is a moderate base such as hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
ion or an alkoxide, the aldol reaction occurs via nucleophilic attack by the resonance-stabilized enolate on the carbonyl group of another molecule. The product is the alkoxide salt of the aldol product. The aldol itself is then formed, and it may then undergo dehydration to give the unsaturated carbonyl compound. The scheme shows a simple mechanism for the base-catalyzed aldol reaction of an aldehyde with itself.
Base-catalyzed aldol reaction (shown using −OCH3 as base)
stoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equ ...
amount of a strong base such as LDA or NaHMDS. In this case, enolate formation is irreversible, and the aldol product is not formed until the metal alkoxide of the aldol product is protonated in a separate workup step.
Zimmerman–Traxler model
More refined forms of the mechanism are known. In 1957, Howard Zimmerman and Marjorie D. Traxler proposed that some aldol reactions have "six-membered transition states having achair conformation
In organic chemistry, cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are ...
." This is now known as the Zimmerman–Traxler model. ''E''-enolates give rise to anti products, whereas ''Z''-enolates give rise to syn products. The factors that control selectivity are the preference for placing substituents equatorially in six-membered transition states and the avoidance of syn-pentane interactions, respectively. E and Z refer to the cis-trans stereochemical relationship between the enolate oxygen bearing the positive counterion and the highest priority group on the alpha carbon. In reality, only some metals such as lithium reliably follow the Zimmerman–Traxler model. Thus, in some cases, the stereochemical outcome of the reaction may be unpredictable.
Crossed-aldol reactant control
The problem of "control" in the aldol addition is best demonstrated by an example. Consider the outcome of this hypothetical reaction:Acidity
The simplest control is if only one of the reactants has acidic protons, and only this molecule forms the enolate. For example, the addition ofdiethyl malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds su ...
into benzaldehyde would produce only one product. Only the malonate has α hydrogens, so it is the nucleophilic partner, whereas the non-enolizeable benzaldehyde can only be the electrophile:
malonic ester synthesis
The malonic ester synthesis is a chemical reaction where diethyl malonate or another ester of malonic acid is alkylated at the carbon alpha (directly adjacent) to both carbonyl groups, and then converted to a substituted acetic acid. The major ...
and acetoacetic ester synthesis Acetoacetic ester synthesis is a chemical reaction where ethyl acetoacetate is alkylated at the α-carbon to both carbonyl groups and then converted into a ketone, or more specifically an α-substituted acetone. This is very similar to malonic ester ...
).
Order of addition
One common solution is to form the enolate of one partner first, and then add the other partner underkinetic control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or ...
.Bal, B.; Buse, C. T.; Smith, K.; Heathcock, C. H.(2SR,3RS)-2,4-Dimethyl-3-Hydroxypentanoic Acid
, '' Org. Synth.'', Coll. Vol. 7, p.185 (1990); Vol. 63, p.89 (1985). Kinetic control means that the forward aldol addition reaction must be significantly faster than the reverse retro-aldol reaction. For this approach to succeed, two other conditions must also be satisfied; it must be possible to quantitatively form the enolate of one partner, and the forward aldol reaction must be significantly faster than the transfer of the enolate from one partner to another. Common kinetic control conditions involve the formation of the enolate of a ketone with LDA at −78 °C, followed by the slow addition of an aldehyde.
Enolates
Formation
The enolate may be formed by using a strong base ("hard conditions", pathway 1 below) or using a Lewis acid and a weak base ("soft conditions", pathway 2 below):deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju. ...
to occur, the stereoelectronic requirement is that the alpha-C-H sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of s ...
must be able to overlap with the pi* orbital of the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
:

Geometry
Extensive studies have been performed on the formation of enolates. It is possible to generate, in most cases, the desired enolate geometry:ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides a ...
s, most enolization conditions give ''E'' enolates. The addition of HMPA is known to reverse the stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non-stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
of deprotonation.
monomer
In chemistry, a monomer ( ; '' mono-'', "one" + ''-mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
ic or oligomeric in nature; nonetheless, the Ireland model remains a useful tool for understanding enolates.
In the Ireland model, the deprotonation is assumed to proceed by a six-membered or cyclic monomeric transition state. The larger of the two substituents on the electrophile (in the case above, methyl is larger than proton) adopts an equatorial disposition in the favored transition state, leading to a preference for E enolates. The model clearly fails in many cases; for example, if the solvent mixture is changed from THF to 23% HMPA-THF (as seen above), the enolate geometry is reversed, which is inconsistent with this model and its cyclic transition state.
Regiochemistry
If an unsymmetrical ketone is subjected to base, it has the potential to form two regioisomeric enolates (ignoring enolate geometry). For example:Stereoselectivity
The aldol reaction is particularly useful because two new stereogenic centers are generated in one reaction. The ''syn''/''anti'' convention is commonly used to denote the relative stereochemistry at the α- and β-carbon.Enolate geometry
There is no significant difference between the level of stereoinduction observed with ''E'' and ''Z'' enolates. Each alkene geometry leads primarily to one specific relative stereochemistry in the product, ''E'' giving ''anti'' and ''Z'' giving ''syn'':Metal ion
The enolate metal cation may play a large role in determining the level of stereoselectivity in the aldol reaction. Boron is often used because itsbond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
s are significantly shorter than that of metals such as lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
, or magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
.
transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
and gives greater stereoselectivity in the reaction. Thus the above reaction gives a ''syn:anti'' ratio of 80:20 using a lithium enolate compared to 97:3 using a bibutylboron enolate.
Alpha stereocenter on the enolate
The aldol reaction may exhibit "substrate-based stereocontrol", in which existing chirality on either reactant influences the stereochemical outcome of the reaction. This has been extensively studied, and in many cases, one can predict the sense of asymmetric induction, if not the absolute level ofdiastereoselectivity
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
. If the enolate contains a stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups c ...
in the alpha position, excellent stereocontrol may be realized.
epimer
In stereochemistry, an epimer is one of a pair of diastereomers. The two epimers have opposite configuration at only one stereogenic center out of at least two. All other stereogenic centers in the molecules are the same in each. Epimerization i ...
ized; in reality, the opposite diastereoface of the aldehyde would have been attacked. In both cases, the 1,3-syn diastereomer is favored. There are many examples of this type of stereocontrol:
Alpha stereocenter on the electrophile
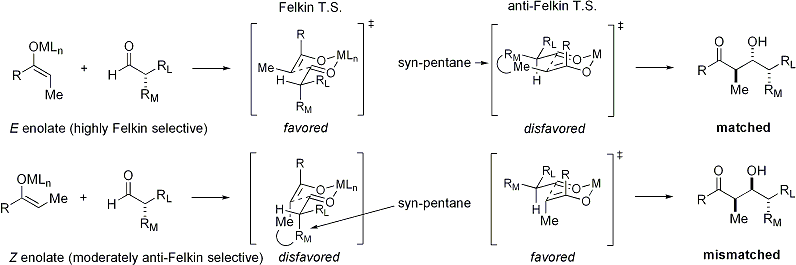
When enolates attacks aldehydes with an alpha stereocenter, excellent stereocontrol is also possible. The general observation is that ''E'' enolates exhibit Felkin diastereoface selection, while ''Z'' enolates exhibit anti-Felkin selectivity. The general modelEvans D. A. ''et al.'' ''Top. Stereochem.'' 1982, ''13'', 1–115. (Review) is presented below: Since ''Z'' enolates must react through a
Since ''Z'' enolates must react through a transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
that contains either a destabilizing syn-pentane interaction or an anti-Felkin rotamer
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a mole ...
, ''Z''-enolates exhibit lower levels of diastereoselectivity in this case. Some examples are presented below:

Unified model of stereoinduction
If both the enolate and the aldehyde contain pre-existing chirality, then the outcome of the "double stereodifferentiating" aldol reaction may be predicted using a merged stereochemical model that takes into account the enolate facial bias, enolate geometry, and aldehyde facial bias. Several examples of the application of this model are given below:
Evans' oxazolidinone chemistry
A widely used method is the Evans'acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC ...
oxazolidinone
2-Oxazolidone is a heterocyclic organic compound containing both nitrogen and oxygen in a 5-membered ring.
Oxazolidinones
Evans auxiliaries
Oxazolidinones are a class of compounds containing 2-oxazolidone in the structure. In chemistry, they are ...
method.Gage J. R.; Evans D. A.Diastereoselective Aldol Condensation Using A Chiral Oxazolidinone Auxiliary: (2S*,3S*)-3-Hydroxy-3-Phenyl-2-Methylpropanoic Acid
,
Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and ex ...
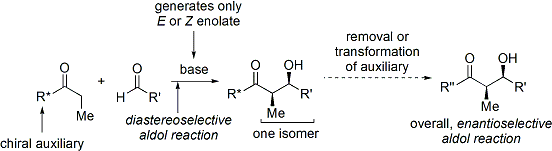
, Coll. Vol. 8, p.339 (1993); Vol. 68, p.83 (1990). Developed in the late 1970s and 1980s by David A. Evans and coworkers, the method works by temporarily creating a chiral enolate by appending a chiral auxiliary
In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the ...
. The pre-existing chirality from the auxiliary is then transferred to the aldol adduct by performing a diastereoselective aldol reaction. Upon subsequent removal of the auxiliary, the desired aldol stereoisomer is revealed.
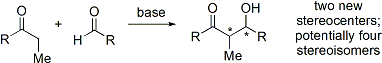

 In the case of the Evans' method, the chiral auxiliary appended is an
In the case of the Evans' method, the chiral auxiliary appended is an oxazolidinone
2-Oxazolidone is a heterocyclic organic compound containing both nitrogen and oxygen in a 5-membered ring.
Oxazolidinones
Evans auxiliaries
Oxazolidinones are a class of compounds containing 2-oxazolidone in the structure. In chemistry, they are ...
, and the resulting carbonyl compound is an imide. A number of oxazolidinones are now readily available in both enantiomeric forms. They are relatively expensive. However, enantiopure oxazolidinones are derived in 2 synthetic steps from comparatively inexpensive amino acids, which means that large-scale syntheses can be made more economical by in-house preparation. This usually involves borohydride mediated reduction of the acid moiety, followed by condensation/cyclisation of the resulting amino alcohol with a simple carbonate ester such as diethylcarbonate.
 The
The acylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in which an acyl group () is added to a compound. The compound providing the acyl group is called the acylating agent.
Because they form a strong electrophile when treated with ...
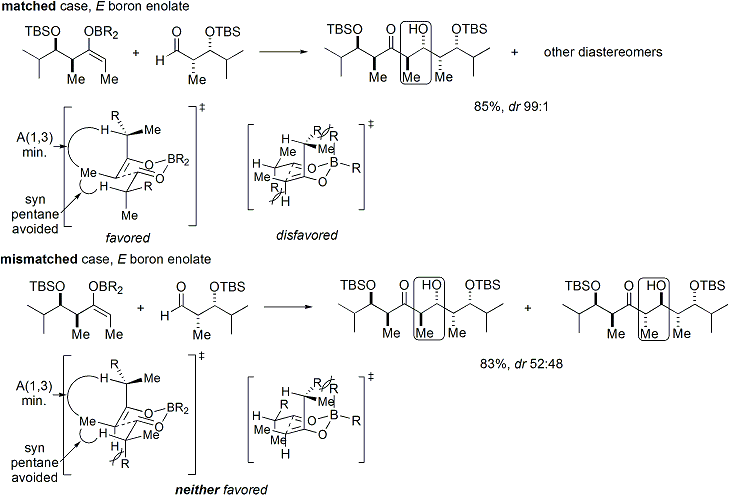
of an oxazolidinone is a convenient procedure, and is informally referred to as "loading done". ''Z''-enolates, leading to syn-aldol adducts, can be reliably formed using boron-mediated soft enolization:
 Often, a single
Often, a single diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
may be obtained by one crystallization of the aldol adduct. However, anti-aldol adducts cannot be obtained reliably with the Evans method. Despite the cost and the limitation to give only ''syn'' adducts, the method's superior reliability, ease of use, and versatility render it the method of choice in many situations. Many methods are available for the cleavage of the auxiliary:
 Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective: and anti selective:
Upon construction of the imide, both syn- and anti-selective aldol addition reactions may be performed, allowing the assemblage of three of the four possible stereoarrays: syn selective: and anti selective:
polyketide
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynth ...
s, a class of natural products that often feature the aldol retron.
Intramolecular reaction
aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
groups or ketone groups in the same molecule. Five- or six-membered , -unsaturated ketone or aldehydes are formed as products. This reaction is an important approach to the formation of carbon-carbon bonds in organic molecules containing ring systems. As an example, under strong basic conditions (e.g. sodium hydroxide), hexane-2,5-dione (compound A in Figure 1) can cyclize via intramolecular aldol reaction to form the 3-methylcyclopent-2-en-1-one (compound B).
The mechanism of the intramolecular aldol reaction involves formation of a key enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
intermediate followed by an intramolecular nucleophilic addition process. First, hydroxide abstracts the α-hydrogen on a terminal carbon to form the enolate. Next, a nucleophilic attack
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
of the enolate on the other keto group forms a new carbon-carbon bond (red) between carbons 2 and 6. At last, usually under heating conditions, the elimination of water molecule yields the cyclized α,β-unsaturated ketone.
Variations and methods
Acetate aldol reactions
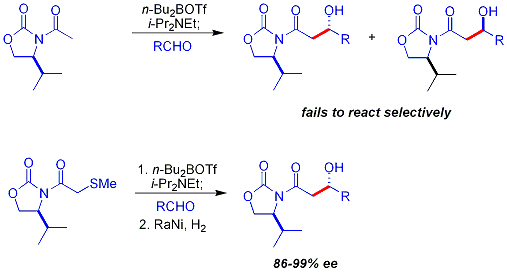
A key limitation to the chiral auxiliary approach described previously is the failure of N-acetyl imides to react selectively. An early approach was to use a temporarythioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A su ...
group:

Mukaiyama aldol reaction
TheMukaiyama aldol reaction
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde or formate. The reaction was discovered by Teruaki Mukaiyama (1927–2018) in 1973. His choice of reactants allows fo ...
is the nucleophilic addition of silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis.
Sy ...
s to aldehydes catalyzed by a Lewis acid such as boron trifluoride (as boron trifluoride etherate) or titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula . It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. is a volatile liquid. Upon contact with humid air, it forms thick clouds ...
. The Mukaiyama aldol reaction does not follow the Zimmerman-Traxler model. Carreira has described particularly useful asymmetric methodology with silyl ketene acetals, noteworthy for its high levels of enantioselectivity and wide substrate scope.
The method works on unbranched aliphatic aldehydes, which are often poor electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carrie ...
s for catalytic, asymmetric processes. This may be due to poor electronic and steric differentiation between their enantiofaces.
 The analogous
The analogous vinylogous
In organic chemistry, vinylogy is the transmission of electronic effects through a conjugated organic bonding system. The concept was introduced in 1926 by Ludwig Claisen to explain the acidic properties of formylacetone and related ketoaldehydes ...
Mukaiyama aldol process can also be rendered catalytic and asymmetric. The example shown below works efficiently for aromatic (but not aliphatic) aldehydes and the mechanism is believed to involve a chiral, metal-bound dienolate.

Crimmins thiazolidinethione aldol
In the Crimmins thiazolidinethione approach a thiazolidinethione performs acetate aldol reactions. and can produce the "Evans syn" or "non-Evans syn" adducts by simply varying the amount of (−)-sparteine. The reaction is believed to proceed via six-membered, titanium-boundtransition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
s, analogous to the proposed transition states for the Evans auxiliary. NOTE: the structure of sparteine shown below is missing a N atom.

Organocatalysis
Chiral secondaryamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen
Hydrogen is the chemical element wi ...
catalysts catalyze some aldol reactions. These secondary amines form transient enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and t ...
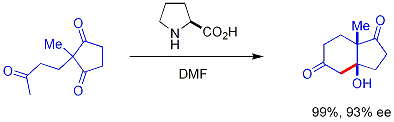
s when exposed to ketones, which may react enantioselectively with suitable aldehyde electrophiles. The amine reacts with the carbonyl to form an enamine, the enamine acts as an enol-like nucleophile, and then the amine is released from the product all—the amine itself is a catalyst. This enamine catalysis method is a type of organocatalysis, since the catalyst is entirely based on a small organic molecule. In a seminal example, proline efficiently catalyzed the cyclization of a triketone:
 This reaction is known as the Hajos-Parrish reaction (also known as the Hajos-Parrish-Eder-Sauer-Wiechert reaction, referring to a contemporaneous report from Schering of the reaction under harsher conditions). Under the Hajos-Parrish conditions only a catalytic amount of proline is necessary (3 mol%). There is no danger of an achiral background reaction because the transient enamine intermediates are much more nucleophilic than their parent ketone enols. This strategy offers a simple way of generating enantioselectivity in reactions without using transition metals, which have the possible disadvantages of being toxic or expensive.
Proline-catalyzed aldol reactions do not show any non-linear effects (the enantioselectivity of the products is directly proportional to the enantiopurity of the catalyst). Combined with
This reaction is known as the Hajos-Parrish reaction (also known as the Hajos-Parrish-Eder-Sauer-Wiechert reaction, referring to a contemporaneous report from Schering of the reaction under harsher conditions). Under the Hajos-Parrish conditions only a catalytic amount of proline is necessary (3 mol%). There is no danger of an achiral background reaction because the transient enamine intermediates are much more nucleophilic than their parent ketone enols. This strategy offers a simple way of generating enantioselectivity in reactions without using transition metals, which have the possible disadvantages of being toxic or expensive.
Proline-catalyzed aldol reactions do not show any non-linear effects (the enantioselectivity of the products is directly proportional to the enantiopurity of the catalyst). Combined with isotopic labelling
Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specif ...
evidence and computational studies, the proposed reaction mechanism for proline-catalyzed aldol reactions is as follows:
 This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can
This strategy allows the otherwise challenging cross-aldol reaction between two aldehydes. In general, cross-aldol reactions between aldehydes are typically challenging because they can polymerize
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many for ...
easily or react unselectively to give a statistical mixture of products. The first example is shown below:
 In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected
In contrast to the preference for syn adducts typically observed in enolate-based aldol additions, these organocatalyzed aldol additions are anti-selective. In many cases, the organocatalytic conditions are mild enough to avoid polymerization. However, selectivity requires the slow syringe-pump controlled addition of the desired electrophilic partner because both reacting partners typically have enolizable protons. If one aldehyde has no enolizable protons or alpha- or beta-branching, additional control can be achieved.
An elegant demonstration of the power of asymmetric organocatalytic aldol reactions was disclosed by MacMillan and coworkers in 2004 in their synthesis of differentially protected carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or m ...
s. While traditional synthetic methods accomplish the synthesis of hexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is C6H12O6, and their molecular weight is 180.156 g/mol.
Hexoses exist in two forms, open-chain or cyclic, that easily convert ...
s using variations of iterative protection-deprotection strategies, requiring 8–14 steps, organocatalysis can access many of the same substrates using an efficient two-step protocol involving the proline-catalyzed dimerization of alpha-oxyaldehydes followed by tandem Mukaiyama aldol cyclization.
 The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha- silyloxy substituents were suitable for this reaction, while aldehydes bearing
The aldol dimerization of alpha-oxyaldehydes requires that the aldol adduct, itself an aldehyde, be inert to further aldol reactions.
Earlier studies revealed that aldehydes bearing alpha-alkyloxy or alpha- silyloxy substituents were suitable for this reaction, while aldehydes bearing Electron-withdrawing group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
s such as acetoxy
In organic chemistry, the acetoxy group (abbr. AcO or OAc; IUPAC name: acetyloxy), is a functional group with the formula and the structure . As the ''-oxy'' suffix implies, it differs from the acetyl group () by the presence of an additio ...
were unreactive. The protected erythrose
Erythrose is a tetrose saccharide with the chemical formula C4H8O4. It has one aldehyde group, and is thus part of the aldose family. The natural isomer is D-erythrose; it is a diastereomer of D -threose.
Erythrose was first isolated in 1849 ...
product could then be converted to four possible sugars via Mukaiyama aldol addition followed by lactol formation. This requires appropriate diastereocontrol in the Mukaiyama aldol addition and the product silyloxycarbenium ion to preferentially cyclize, rather than undergo further aldol reaction. In the end, glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
, mannose, and allose were synthesized:

"Direct" aldol additions
In the usual aldol addition, a carbonyl compound is deprotonated to form the enolate. The enolate is added to an aldehyde or ketone, which forms an alkoxide, which is then protonated on workup. A superior method, in principle, would avoid the requirement for a multistep sequence in favor of a "direct" reaction that could be done in a single process step. One idea is to generate the enolate using a metalcatalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
that is released after the aldol addition mechanism. The general problem is that the addition generates an alkoxide, which is much more basic than the starting materials. This product binds tightly to the metal, preventing it from reacting with additional carbonyl reactants.
 One approach, demonstrated by Evans, is to silylate the aldol adduct. A silicon reagent such as
One approach, demonstrated by Evans, is to silylate the aldol adduct. A silicon reagent such as TMSCl
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. I ...
is added in the reaction, which replaces the metal on the alkoxide, allowing turnover of the metal catalyst. Minimizing the number of reaction steps and amount of reactive chemicals used leads to a cost-effective and industrially useful reaction.

Biological aldol reactions
Examples of aldol reactions in biochemistry include the splitting of fructose-1,6-bisphosphate intodihydroxyacetone
Dihydroxyacetone (; DHA), also known as glycerone, is a simple saccharide (a triose) with formula .
DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, an ...
and glyceraldehyde-3-phosphate in the fourth stage of glycolysis, which is an example of a reverse ("retro") aldol reaction catalyzed by the enzyme aldolase A (also known as fructose-1,6-bisphosphate aldolase).
In the glyoxylate cycle
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrat ...
of plants and some prokaryotes, isocitrate lyase produces glyoxylate and succinate
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin ''succinum'', meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological ro ...
from isocitrate. Following deprotonation of the OH group, isocitrate lyase cleaves isocitrate into the four-carbon succinate and the two-carbon glyoxylate by an aldol cleavage reaction. This cleavage is similar mechanistically to the aldolase A reaction of glycolysis.
See also
* Aldol–Tishchenko reaction *Baylis–Hillman reaction
The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and a carbon electrophile such as an aldehyde. Employing a nucleophilic catalyst, such as a tertiary amine and phosphine, this rea ...
* Ivanov reaction
* Reformatsky reaction
The Reformatsky reaction (sometimes misspelled Reformatskii reaction) is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:
The organozinc reagent, also called a 'Reforma ...
References
External links
* Chem 206, 215 Lecture Notes (2003, 2006) by D. A. Evans,A. G. Myers
A is the first letter of the Latin and English alphabet.
A may also refer to:
Science and technology Quantities and units
* ''a'', a measure for the attraction between particles in the Van der Waals equation
* ''A'' value, a measure of ...
, ''et al.'', Harvard University (pp. 345, 936)
{{Organic reactions
Addition reactions
Carbon-carbon bond forming reactions