Vapor–liquid Equilibrium on:
[Wikipedia]
[Google]
[Amazon]
In
 The preceding equilibrium equations are typically applied for each phase (liquid or vapor) individually, but the result can be plotted in a single diagram. In a binary boiling-point diagram, temperature () (or sometimes pressure) is graphed vs. . At any given temperature (or pressure) where both phases are present, vapor with a certain mole fraction is in equilibrium with liquid with a certain mole fraction. The two mole fractions often differ. These vapor and liquid mole fractions are represented by two points on the same horizontal isotherm (constant ) line. When an entire range of temperatures vs. vapor and liquid mole fractions is graphed, two (usually curved) lines result. The lower one, representing the mole fraction of the boiling liquid at various temperatures, is called the '' bubble point curve''. The upper one, representing the mole fraction of the vapor at various temperatures, is called the ''dew point curve''.
These two curves necessarily meet where the mixture becomes purely one component, namely where (and , pure component 2) or (and , pure component 1). The temperatures at those two points correspond to the boiling points of each of the two pure components.
For certain pairs of substances, the two curves also coincide at some point strictly between and . When they meet, they meet tangently; the dew-point temperature always lies above the boiling-point temperature for a given composition when they are not equal. The meeting point is called an azeotrope for that particular pair of substances. It is characterized by an azeotrope temperature and an azeotropic composition, often expressed as a mole fraction. There can be maximum-boiling azeotropes, where the azeotrope temperature is at a maximum in the boiling curves, or minimum-boiling azeotropes, where the azeotrope temperature is at a minimum in the boiling curves.
If one wants to represent a VLE data for a three-component mixture as a boiling point "diagram", a three-dimensional graph can be used. Two of the dimensions would be used to represent the composition mole fractions, and the third dimension would be the temperature. Using two dimensions, the composition can be represented as an equilateral triangle in which each corner represents one of the pure components. The edges of the triangle represent a mixture of the two components at each end of the edge. Any point inside the triangle represents the composition of a mixture of all three components. The mole fraction of each component would correspond to where a point lies along a line starting at that component's corner and perpendicular to the opposite edge. The bubble point and
The preceding equilibrium equations are typically applied for each phase (liquid or vapor) individually, but the result can be plotted in a single diagram. In a binary boiling-point diagram, temperature () (or sometimes pressure) is graphed vs. . At any given temperature (or pressure) where both phases are present, vapor with a certain mole fraction is in equilibrium with liquid with a certain mole fraction. The two mole fractions often differ. These vapor and liquid mole fractions are represented by two points on the same horizontal isotherm (constant ) line. When an entire range of temperatures vs. vapor and liquid mole fractions is graphed, two (usually curved) lines result. The lower one, representing the mole fraction of the boiling liquid at various temperatures, is called the '' bubble point curve''. The upper one, representing the mole fraction of the vapor at various temperatures, is called the ''dew point curve''.
These two curves necessarily meet where the mixture becomes purely one component, namely where (and , pure component 2) or (and , pure component 1). The temperatures at those two points correspond to the boiling points of each of the two pure components.
For certain pairs of substances, the two curves also coincide at some point strictly between and . When they meet, they meet tangently; the dew-point temperature always lies above the boiling-point temperature for a given composition when they are not equal. The meeting point is called an azeotrope for that particular pair of substances. It is characterized by an azeotrope temperature and an azeotropic composition, often expressed as a mole fraction. There can be maximum-boiling azeotropes, where the azeotrope temperature is at a maximum in the boiling curves, or minimum-boiling azeotropes, where the azeotrope temperature is at a minimum in the boiling curves.
If one wants to represent a VLE data for a three-component mixture as a boiling point "diagram", a three-dimensional graph can be used. Two of the dimensions would be used to represent the composition mole fractions, and the third dimension would be the temperature. Using two dimensions, the composition can be represented as an equilateral triangle in which each corner represents one of the pure components. The edges of the triangle represent a mixture of the two components at each end of the edge. Any point inside the triangle represents the composition of a mixture of all three components. The mole fraction of each component would correspond to where a point lies along a line starting at that component's corner and perpendicular to the opposite edge. The bubble point and
 The tendency of a given chemical species to partition itself
preferentially between liquid and vapor phases is the Henry's law constant. There can be VLE data for mixtures of four or more components, but such a boiling-point diagram is hard to show in either tabular or graphical form. For such multi-component mixtures, as well as binary
mixtures, the vapor–liquid equilibrium data are represented in terms of values ( vapor–liquid distribution ratios) defined by
:
where and are the mole fractions of component in the phases and respectively.
For Raoult's law
:
For modified Raoult's law
:
where is the
The tendency of a given chemical species to partition itself
preferentially between liquid and vapor phases is the Henry's law constant. There can be VLE data for mixtures of four or more components, but such a boiling-point diagram is hard to show in either tabular or graphical form. For such multi-component mixtures, as well as binary
mixtures, the vapor–liquid equilibrium data are represented in terms of values ( vapor–liquid distribution ratios) defined by
:
where and are the mole fractions of component in the phases and respectively.
For Raoult's law
:
For modified Raoult's law
:
where is the 
 For binary mixtures, the ratio of the values for the two components is called the relative volatility denoted by
:
which is a measure of the relative ease or difficulty of separating the two components. Large-scale industrial distillation is rarely undertaken if the relative volatility is less than 1.05 with the volatile component being and the less volatile component being .
values are widely used in the design calculations of
For binary mixtures, the ratio of the values for the two components is called the relative volatility denoted by
:
which is a measure of the relative ease or difficulty of separating the two components. Large-scale industrial distillation is rarely undertaken if the relative volatility is less than 1.05 with the volatile component being and the less volatile component being .
values are widely used in the design calculations of
 For each component in a binary mixture, one could make a vapor–liquid equilibrium diagram. Such a diagram would graph liquid mole fraction on a horizontal axis and vapor mole fraction on a vertical axis. In such VLE diagrams, liquid mole fractions for components 1 and 2 can be represented as and respectively, and vapor mole fractions of the corresponding components are commonly represented as and . Similarly for binary mixtures in these VLE diagrams:
: and
Such VLE diagrams are square with a diagonal line running from the () corner to the () corner for reference.
These types of VLE diagrams are used in the
For each component in a binary mixture, one could make a vapor–liquid equilibrium diagram. Such a diagram would graph liquid mole fraction on a horizontal axis and vapor mole fraction on a vertical axis. In such VLE diagrams, liquid mole fractions for components 1 and 2 can be represented as and respectively, and vapor mole fractions of the corresponding components are commonly represented as and . Similarly for binary mixtures in these VLE diagrams:
: and
Such VLE diagrams are square with a diagonal line running from the () corner to the () corner for reference.
These types of VLE diagrams are used in the
Distillation Principals
by Ming T. Tham, University of Newcastle upon Tyne (scroll down to Relative Volatility)
Introduction to Distillation: Vapor Liquid Equilibria
(Chemical Engineering Dept., Prof. Richard Rowley, Brigham Young University)
NIST Standard Reference Database 103b
(Describes the extensive VLE database available from
Some VLE data sets and diagrams for mixtures of 30 common components
a small subset of the Dortmund Data Bank
Where can I get the vapor-liquid phase equilibrium data?
Reference to the various phase equilibrium data sources
Can. J. Chem. Eng. ternary and multicomponent systems from binary ones
* George Schlowsky, Alan Erickson, and Thomas A. Schafer, Modular Process Systems, Inc.
Operations & Maintenance - Generating your own VLE Data
Chemical Engineering, March 1995, McGraw-Hill, Inc.
thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws ...
and chemical engineering
Chemical engineering is an engineering field which deals with the study of operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials in ...
, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species
A chemical species is a chemical substance or ensemble composed of chemically identical molecular entities that can explore the same set of molecular energy levels on a characteristic or delineated time scale. These energy levels determine the wa ...
between the vapor phase and a liquid phase.
The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed pha ...
, which will be a partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
(a part of the total gas pressure) if any other gas(es) are present with the vapor. The equilibrium vapor pressure of a liquid is in general strongly dependent on temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
. At vapor–liquid equilibrium, a liquid with individual components in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components have certain values depending on all of the liquid component concentrations and the temperature. The converse is also true: if a vapor with components at certain concentrations or partial pressures is in vapor–liquid equilibrium with its liquid, then the component concentrations in the liquid will be determined dependent on the vapor concentrations and on the temperature. The equilibrium concentration of each component in the liquid phase is often different from its concentration (or vapor pressure) in the vapor phase, but there is a relationship. The VLE concentration data can be determined experimentally or approximated with the help of theories such as Raoult's law, Dalton's law, and Henry's law.
Such vapor–liquid equilibrium information is useful in designing columns for distillation
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the he ...
, especially fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
, which is a particular specialty of chemical engineers. Distillation is a process used to separate or partially separate components in a mixture by boiling (vaporization) followed by condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapo ...
. Distillation takes advantage of differences in concentrations of components in the liquid and vapor phases.
In mixtures containing two or more components, the concentrations of each component are often expressed as mole fractions. The mole fraction of a given component of a mixture in a particular phase (either the vapor or the liquid phase) is the number of moles Moles can refer to:
* Moles de Xert, a mountain range in the Baix Maestrat comarca, Valencian Community, Spain
*The Moles (Australian band)
*The Moles, alter ego of Scottish band Simon Dupree and the Big Sound
People
* Abraham Moles, French engin ...
of that component in that phase divided by the total number of moles of all components in that phase.
Binary mixtures are those having two components. Three-component mixtures are called ternary mixtures. There can be VLE data for mixtures with even more components, but such data is often hard to show graphically. VLE data is a function of the total pressure, such as 1 atm or at the pressure the process is conducted at.
When a temperature is reached such that the sum of the equilibrium vapor pressures of the liquid components becomes equal to the total pressure of the system (it is otherwise smaller), then vapor bubbles generated from the liquid begin to displace the gas that was maintaining the overall pressure, and the mixture is said to boil. This temperature is called the ''boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding env ...
'' of the liquid mixture at the given pressure. (It is assumed that the total pressure is held steady by adjusting the total volume of the system to accommodate the specific volume changes that accompany boiling.) The boiling point at an overall pressure of 1 atm is called the '' normal boiling point'' of the liquid mixture.
Thermodynamic description of vapor–liquid equilibrium
The field ofthermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws ...
describes when vapor–liquid equilibrium is possible, and its properties. Much of the analysis depends on whether the vapor and liquid consist of a single component, or if they are mixtures.
Pure (single-component) systems
If the liquid and vapor are pure, in that they consist of only one molecular component and no impurities, then the equilibrium state between the two phases is described by the following equations: :; :; and : where and are thepressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
s within the liquid and vapor, and are the temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied o ...
s within the liquid and vapor, and and are the molar Gibbs free energies (units of energy per amount of substance) within the liquid and vapor, respectively. In other words, the temperature, pressure and molar Gibbs free energy are the same between the two phases when they are at equilibrium.
An equivalent, more common way to express the vapor–liquid equilibrium condition in a pure system is by using the concept of fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas whic ...
. Under this view, equilibrium is described by the following equation:
:
where and are the fugacities of the liquid and vapor, respectively, at the system temperature and pressure . It is often convenient to use the quantity , the dimensionless ''fugacity coefficient'', which is 1 for an ideal gas.
Multicomponent systems
In a multicomponent system, where the vapor and liquid consist of more than one type of compounds, describing the equilibrium state is more complicated. For all components in the system, the equilibrium state between the two phases is described by the following equations: :; :; and : where and are the temperature and pressure for each phase, and and are thepartial molar Gibbs free energy
In thermodynamics, a partial molar property is a quantity which describes the variation of an extensive property of a solution or mixture with changes in the molar composition of the mixture at constant temperature and pressure. It is the part ...
also called chemical potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a speci ...
(units of energy per amount of substance) within the liquid and vapor, respectively, for each phase. The partial molar Gibbs free energy is defined by:
:
where is the ( extensive) Gibbs free energy, and is the amount of substance of component .
Boiling-point diagrams
Binary mixture VLE data at a certain overall pressure, such as 1 atm, showing mole fraction vapor and liquid concentrations when boiling at various temperatures can be shown as a two-dimensional graph called a boiling-point diagram. The mole fraction of component 1 in the mixture can be represented by the symbol . The mole fraction of component 2, represented by , is related to in a binary mixture as follows: : In multi-component mixtures in general with n components, this becomes: :dew point
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will ...
data would become curved surfaces inside a triangular prism, which connect the three boiling points on the vertical temperature "axes". Each face of this triangular prism would represent a two-dimensional boiling-point diagram for the corresponding binary mixture. Due to their three-dimensional complexity, such boiling-point diagrams are rarely seen. Alternatively, the three-dimensional curved surfaces can be represented on a two-dimensional graph by the use of curved isotherm lines at graduated intervals, similar to iso-altitude lines on a map. Two sets of such isotherm lines are needed on such a two-dimensional graph: one set for the bubble point surface and another set for the dew point surface.
''K'' values and relative volatility values
 The tendency of a given chemical species to partition itself
preferentially between liquid and vapor phases is the Henry's law constant. There can be VLE data for mixtures of four or more components, but such a boiling-point diagram is hard to show in either tabular or graphical form. For such multi-component mixtures, as well as binary
mixtures, the vapor–liquid equilibrium data are represented in terms of values ( vapor–liquid distribution ratios) defined by
:
where and are the mole fractions of component in the phases and respectively.
For Raoult's law
:
For modified Raoult's law
:
where is the
The tendency of a given chemical species to partition itself
preferentially between liquid and vapor phases is the Henry's law constant. There can be VLE data for mixtures of four or more components, but such a boiling-point diagram is hard to show in either tabular or graphical form. For such multi-component mixtures, as well as binary
mixtures, the vapor–liquid equilibrium data are represented in terms of values ( vapor–liquid distribution ratios) defined by
:
where and are the mole fractions of component in the phases and respectively.
For Raoult's law
:
For modified Raoult's law
:
where is the activity coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same ...
, is the partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
and is the pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
.
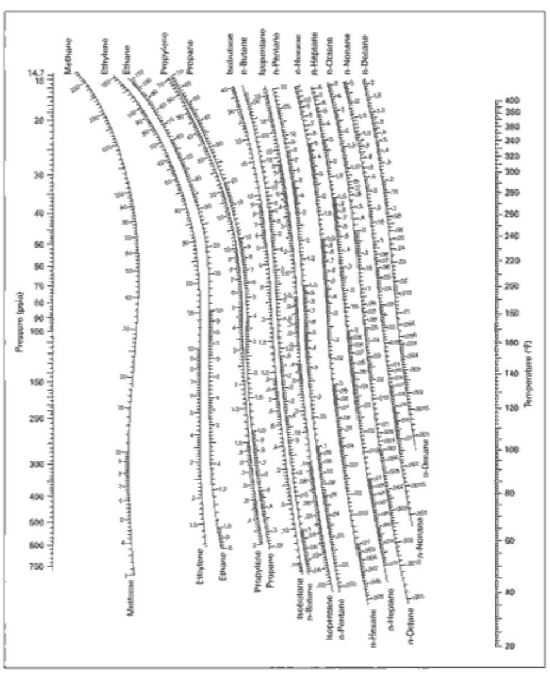
The values of the ratio are correlated empirically or theoretically in terms of temperature, pressure and phase compositions in the form of equations, tables or graph such as the DePriester charts.DePriester, C.L., ''Chem. Eng. Prog. Symposium Series'', 7, 49, pages 1–43
continuous distillation
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams. Distillation is the sep ...
columns for distilling multicomponent mixtures.
Vapor–liquid equilibrium diagrams
McCabe–Thiele method
The McCabe–Thiele method is a chemical engineering technique for the analysis of binary distillation. It uses the fact that the composition at each theoretical tray (or equilibrium stage) is completely determined by the mole fraction of one of ...
to determine the number of equilibrium stages (or theoretical plates) needed to distill a given composition binary feed mixture into one distillate fraction and one bottoms fraction. Corrections can also be made to take into account the incomplete efficiency of each tray in a distillation column when compared to a theoretical plate.
Raoult's law
At boiling and higher temperatures the sum of the individual component partial pressures becomes equal to the overall pressure, which can symbolized as . Under such conditions, Dalton's law would be in effect as follows: :''P''tot = ''P''1 + ''P''2 + ... Then for each component in the vapor phase: :''y''1 = ''P''1 / ''P''tot, ''y''2 = ''P''2 / ''P''tot, ... etc. where ''P''1 = partial pressure of component 1, = partial pressure of component 2, etc. Raoult's law is approximately valid for mixtures of components between which there is very little interaction other than the effect of dilution by the other components. Examples of such mixtures includes mixtures ofalkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in wh ...
s, which are non-polar
Polar may refer to:
Geography
Polar may refer to:
* Geographical pole, either of two fixed points on the surface of a rotating body or planet, at 90 degrees from the equator, based on the axis around which a body rotates
*Polar climate, the cli ...
, relatively inert compounds in many ways, so there is little attraction or repulsion between the molecules. Raoult's law states that for components 1, 2, etc. in a mixture:
:''P''1 = ''x''1 ''P'' o1, ''P''2 = ''x''2 ''P'' o2, ... etc.
where ''P'' o1, P o2, etc. are the vapor pressures of components 1, 2, etc. when they are pure, and ''x''1, ''x''2, etc. are mole fractions of the corresponding component in the liquid.
Recall from the first section that vapor pressures of liquids are very dependent on temperature. Thus the ''P''o pure vapor pressures for each component are a function of temperature (''T''): For example, commonly for a pure liquid component, the Clausius–Clapeyron relation may be used to approximate how the vapor pressure varies as a function of temperature. This makes each of the partial pressures dependent on temperature also regardless of whether Raoult's law applies or not. When Raoult's law is valid these expressions become:
:''P''1''T'' = ''x''1 ''P'' o1''T'', ''P''2''T'' = ''x''2 ''P'' o2''T'', ... etc.
At boiling temperatures if Raoult's law applies, the total pressure becomes:
:''P''tot = ''x''1 ''P'' o1''T'' + ''x''2 ''P'' o2''T'' + ... etc.
At a given ''P''tot such as 1 atm and a given liquid composition, ''T'' can be solved for to give the liquid mixture's boiling point or bubble point, although the solution for ''T'' may not be mathematically analytical (i.e., may require a numerical solution or approximation). For a binary mixture at a given ''P''tot, the bubble point ''T'' can become a function of ''x''1 (or ''x''2) and this function can be shown on a two-dimensional graph like a binary boiling point diagram.
At boiling temperatures if Raoult's law applies, a number of the preceding equations in this section can be combined to give the following expressions for vapor mole fractions as a function of liquid mole fractions and temperature:
:''y''1 = ''x''1 ''P'' o1''T'' / ''P''tot, ''y''2 = ''x''2 ''P'' o2''T'' / ''P''tot, ... etc.
Once the bubble point ''Ts as a function of liquid composition in terms of mole fractions have been determined, these values can be inserted into the above equations to obtain corresponding vapor compositions in terms of mole fractions. When this is finished over a complete range of liquid mole fractions and their corresponding temperatures, one effectively obtains a temperature ''T'' function of vapor composition mole fractions. This function effectively acts as the dew point ''T'' function of vapor composition.
In the case of a binary mixture, ''x''2 = 1 − ''x''1 and the above equations can be expressed as:
:''y''1 = ''x''1 ''P'' o1''T'' / ''P''tot, and
:''y''2 = (1 − ''x''1) ''P'' o2''T'' / ''P''tot
For many kinds of mixtures, particularly where there is interaction between components beyond simply the effects of dilution, Raoult's law does not work well for determining the shapes of the curves in the boiling point or VLE diagrams. Even in such mixtures, there are usually still differences in the vapor and liquid equilibrium concentrations at most points, and distillation is often still useful for separating components at least partially. For such mixtures, empirical data is typically used in determining such boiling point and VLE diagrams. Chemical engineers have done a significant amount of research trying to develop equations for correlating and/or predicting VLE data for various kinds of mixtures which do not obey Raoult's law well.
See also
*Continuous distillation
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams. Distillation is the sep ...
* Dortmund Data Bank (includes a collection of VLE data)
* Fenske equation
* Flash evaporation
* DECHEMA model
*Hand boiler
A hand boiler or (less commonly) love meter is a glass sculpture used as an experimental tool to demonstrate vapour-liquid equilibrium, or as a collector's item to whimsically "measure love." It consists of a lower bulb containing a volatile liq ...
*Van Laar equation
The Van Laar equation is a thermodynamic activity model, which was developed by Johannes van Laar in 1910-1913, to describe phase equilibria of liquid mixtures. The equation was derived from the Van der Waals equation. The original van der Waals pa ...
*Margules activity model The Margules activity model is a simple thermodynamic model for the excess Gibbs free energy of a liquid mixture introduced in 1895 by Max Margules. After Lewis had introduced the concept of the activity coefficient, the model could be used to der ...
* Pervaporation
* Supercooling
*Superheated steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured.
Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of ...
External links
Distillation Principals
by Ming T. Tham, University of Newcastle upon Tyne (scroll down to Relative Volatility)
Introduction to Distillation: Vapor Liquid Equilibria
(Chemical Engineering Dept., Prof. Richard Rowley, Brigham Young University)
NIST Standard Reference Database 103b
(Describes the extensive VLE database available from
NIST
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical sci ...
)
Some VLE data sets and diagrams for mixtures of 30 common components
a small subset of the Dortmund Data Bank
Where can I get the vapor-liquid phase equilibrium data?
Reference to the various phase equilibrium data sources
Can. J. Chem. Eng. ternary and multicomponent systems from binary ones
* George Schlowsky, Alan Erickson, and Thomas A. Schafer, Modular Process Systems, Inc.
Operations & Maintenance - Generating your own VLE Data
Chemical Engineering, March 1995, McGraw-Hill, Inc.
References
{{DEFAULTSORT:Vapor-Liquid Equilibrium Chemical engineering thermodynamics Equilibrium chemistry Distillation Phases of matter