Ubiquitin Ligases on:
[Wikipedia]
[Google]
[Amazon]
A ubiquitin ligase (also called an E3 ubiquitin ligase) is a
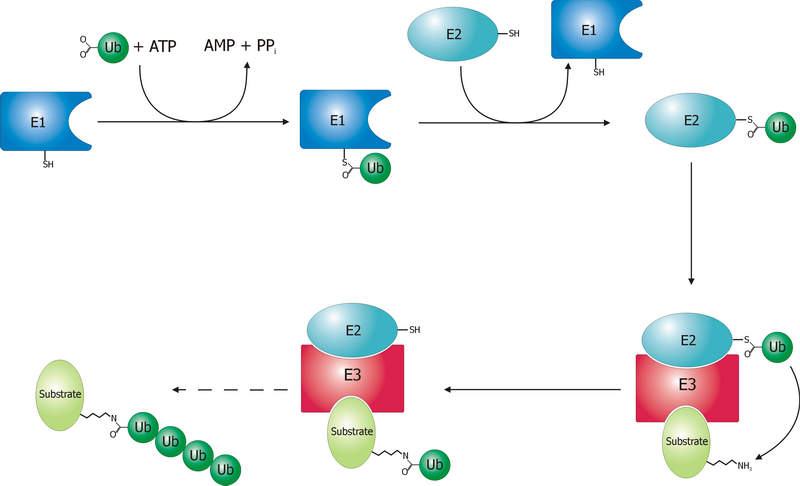 The ubiquitin ligase is referred to as an E3, and operates in conjunction with an E1 ubiquitin-activating enzyme and an E2 ubiquitin-conjugating enzyme. There is one major E1 enzyme, shared by all ubiquitin ligases, that uses ATP to activate
The ubiquitin ligase is referred to as an E3, and operates in conjunction with an E1 ubiquitin-activating enzyme and an E2 ubiquitin-conjugating enzyme. There is one major E1 enzyme, shared by all ubiquitin ligases, that uses ATP to activate
 Ubiquitin signaling relies on the diversity of ubiquitin tags for the specificity of its message. A protein can be tagged with a single ubiquitin molecule (monoubiquitylation), or variety of different chains of ubiquitin molecules (polyubiquitylation). E3 ubiquitin ligases catalyze polyubiquitination events much in the same way as the single ubiquitylation mechanism, using instead a lysine residue from a ubiquitin molecule currently attached to substrate protein to attack the C-terminus of a new ubiquitin molecule. For example, a common 4-ubiquitin tag, linked through the lysine at position 48 (K48) recruits the tagged protein to the proteasome, and subsequent degradation. However, all seven of the ubiquitin lysine residues (K6, K11, K27, K29, K33, K48, and K63), as well as the N-terminal methionine are used in chains in vivo.
Monoubiquitination has been linked to membrane protein endocytosis pathways. For example, phosphorylation of the Tyrosine at position 1045 in the
Ubiquitin signaling relies on the diversity of ubiquitin tags for the specificity of its message. A protein can be tagged with a single ubiquitin molecule (monoubiquitylation), or variety of different chains of ubiquitin molecules (polyubiquitylation). E3 ubiquitin ligases catalyze polyubiquitination events much in the same way as the single ubiquitylation mechanism, using instead a lysine residue from a ubiquitin molecule currently attached to substrate protein to attack the C-terminus of a new ubiquitin molecule. For example, a common 4-ubiquitin tag, linked through the lysine at position 48 (K48) recruits the tagged protein to the proteasome, and subsequent degradation. However, all seven of the ubiquitin lysine residues (K6, K11, K27, K29, K33, K48, and K63), as well as the N-terminal methionine are used in chains in vivo.
Monoubiquitination has been linked to membrane protein endocytosis pathways. For example, phosphorylation of the Tyrosine at position 1045 in the
 A degron can be converted into its active form by a
A degron can be converted into its active form by a
Quips article describing E3 Ligase function
a
PDBe
* * {{DEFAULTSORT:Ubiquitin Ligase EC 6.3 Post-translational modification
protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
that recruits an E2 ubiquitin-conjugating enzyme
Ubiquitin-conjugating enzymes, also known as E2 enzymes and more rarely as ''ubiquitin-carrier enzymes'', perform the second step in the ubiquitination reaction that targets a protein for degradation via the proteasome. The ubiquitination process ...
that has been loaded with ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
, recognizes a protein substrate, and assists or directly catalyzes the transfer of ubiquitin from the E2 to the protein substrate. In simple and more general terms, the ligase enables movement of ubiquitin from a ubiquitin carrier to another thing (the substrate) by some mechanism. The ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
, once it reaches its destination, ends up being attached by an isopeptide bond to a lysine residue, which is part of the target protein. E3 ligases interact with both the target protein and the E2 enzyme, and so impart substrate specificity to the E2. Commonly, E3s polyubiquitinate their substrate with Lys48-linked chains of ubiquitin, targeting the substrate for destruction by the proteasome. However, many other types of linkages are possible and alter a protein's activity, interactions, or localization. Ubiquitination by E3 ligases regulates diverse areas such as cell trafficking, DNA repair, and signaling and is of profound importance in cell biology. E3 ligases are also key players in cell cycle control, mediating the degradation of cyclins
Cyclin is a family of proteins that controls the progression of a cell through the cell cycle by activating cyclin-dependent kinase (CDK) enzymes or group of enzymes required for synthesis of cell cycle.
Etymology
Cyclins were originally di ...
, as well as cyclin dependent kinase inhibitor proteins. The human genome encodes over 600 putative E3 ligases, allowing for tremendous diversity in substrates.
Ubiquitination system
 The ubiquitin ligase is referred to as an E3, and operates in conjunction with an E1 ubiquitin-activating enzyme and an E2 ubiquitin-conjugating enzyme. There is one major E1 enzyme, shared by all ubiquitin ligases, that uses ATP to activate
The ubiquitin ligase is referred to as an E3, and operates in conjunction with an E1 ubiquitin-activating enzyme and an E2 ubiquitin-conjugating enzyme. There is one major E1 enzyme, shared by all ubiquitin ligases, that uses ATP to activate ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
for conjugation
Conjugation or conjugate may refer to:
Linguistics
* Grammatical conjugation, the modification of a verb from its basic form
* Emotive conjugation or Russell's conjugation, the use of loaded language
Mathematics
* Complex conjugation, the chang ...
and transfers it to an E2 enzyme. The E2 enzyme interacts with a specific E3 partner and transfers the ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
to the target protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
. The E3, which may be a multi-protein complex, is, in general, responsible for targeting ubiquitination to specific substrate proteins.
The ubiquitylation reaction proceeds in three or four steps depending on the mechanism of action of the E3 ubiquitin ligase. In the conserved first step, an E1 cysteine residue attacks the ATP-activated C-terminal glycine on ubiquitin, resulting in a thioester
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by t ...
Ub-S-E1 complex. The energy from ATP and diphosphate hydrolysis drives the formation of this reactive thioester, and subsequent steps are thermoneutral. Next, a transthiolation reaction occurs, in which an E2 cysteine residue attacks and replaces the E1. HECT domain
In molecular biology, the HECT domain is a protein domain found in ubiquitin-protein ligases. The name HECT comes from 'Homologous to the E6-AP Carboxyl Terminus'. Proteins containing this domain at the C terminus include ubiquitin-protein ligase ...
type E3 ligases will have one more transthiolation reaction to transfer the ubiquitin molecule onto the E3, whereas the much more common RING finger domain
In molecular biology, a RING (short for Really Interesting New Gene) finger domain is a protein structural domain of zinc finger type which contains a C3HC4 amino acid motif which binds two zinc cations (seven cysteines and one histidine arrange ...
type ligases transfer ubiquitin directly from E2 to the substrate. The final step in the first ubiquitylation event is an attack from the target protein lysine amine group, which will remove the cysteine, and form a stable isopeptide bond. One notable exception to this is p21 protein, which appears to be ubiquitylated using its N-terminal amine, thus forming a peptide bond with ubiquitin.
Ubiquitin ligase families
Humans have an estimated 500-1000 E3 ligases, which impart substrate specificity onto the E1 and E2. The E3 ligases are classified into four families: HECT, RING-finger, U-box, and PHD-finger. The RING-finger E3 ligases are the largest family and contain ligases such as theanaphase-promoting complex
Anaphase-promoting complex (also called the cyclosome or APC/C) is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cull ...
(APC) and the SCF complex
Skp, Cullin, F-box containing complex (or SCF complex) is a multi-protein E3 ubiquitin ligase complex that catalyzes the ubiquitination of proteins destined for 26S proteasomal degradation. Along with the anaphase-promoting complex, SCF has impo ...
(Skp1
S-phase kinase-associated protein 1 is an enzyme that in humans is encoded by the ''SKP1'' gene.
This gene encodes a protein that is a member of the SCF ubiquitin ligase protein complex. It binds to F-box proteins (proteins containing an F-box ...
-Cullin
Cullins are a family of hydrophobic scaffold proteins which provide support for ubiquitin ligases (E3). All eukaryotes appear to have cullins. They combine with RING proteins to form ''Cullin-RING ubiquitin ligases'' (CRLs) that are highly dive ...
-F-box protein complex). SCF complexes consist of four proteins: Rbx1, Cul1, Skp1, which are invariant among SCF complexes, and an F-box protein, which varies. Around 70 human F-box proteins have been identified. F-box proteins contain an F-box, which binds the rest of the SCF complex, and a substrate binding domain, which gives the E3 its substrate specificity.
Mono- and poly-ubiquitylation
 Ubiquitin signaling relies on the diversity of ubiquitin tags for the specificity of its message. A protein can be tagged with a single ubiquitin molecule (monoubiquitylation), or variety of different chains of ubiquitin molecules (polyubiquitylation). E3 ubiquitin ligases catalyze polyubiquitination events much in the same way as the single ubiquitylation mechanism, using instead a lysine residue from a ubiquitin molecule currently attached to substrate protein to attack the C-terminus of a new ubiquitin molecule. For example, a common 4-ubiquitin tag, linked through the lysine at position 48 (K48) recruits the tagged protein to the proteasome, and subsequent degradation. However, all seven of the ubiquitin lysine residues (K6, K11, K27, K29, K33, K48, and K63), as well as the N-terminal methionine are used in chains in vivo.
Monoubiquitination has been linked to membrane protein endocytosis pathways. For example, phosphorylation of the Tyrosine at position 1045 in the
Ubiquitin signaling relies on the diversity of ubiquitin tags for the specificity of its message. A protein can be tagged with a single ubiquitin molecule (monoubiquitylation), or variety of different chains of ubiquitin molecules (polyubiquitylation). E3 ubiquitin ligases catalyze polyubiquitination events much in the same way as the single ubiquitylation mechanism, using instead a lysine residue from a ubiquitin molecule currently attached to substrate protein to attack the C-terminus of a new ubiquitin molecule. For example, a common 4-ubiquitin tag, linked through the lysine at position 48 (K48) recruits the tagged protein to the proteasome, and subsequent degradation. However, all seven of the ubiquitin lysine residues (K6, K11, K27, K29, K33, K48, and K63), as well as the N-terminal methionine are used in chains in vivo.
Monoubiquitination has been linked to membrane protein endocytosis pathways. For example, phosphorylation of the Tyrosine at position 1045 in the Epidermal Growth Factor Receptor
The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is a transmembrane protein that is a receptor for members of the epidermal growth factor family (EGF family) of extracellular protein ligands.
The epidermal growth factor rece ...
(EGFR) can recruit the RING type E3 ligase c-Cbl, via an SH2 domain
The SH2 (Src Homology 2) domain is a structurally conserved protein domain contained within the Src oncoprotein and in many other intracellular signal-transducing proteins. SH2 domains allow proteins containing those domains to dock to phosphor ...
. C-Cbl monoubiquitylates EGFR, signaling for its internalization and trafficking to the lysosome.
Monoubiquitination also can regulate cytosolic protein localization. For example, the E3 ligase MDM2 ubiquitylates p53
p53, also known as Tumor protein P53, cellular tumor antigen p53 (UniProt name), or transformation-related protein 53 (TRP53) is a regulatory protein that is often mutated in human cancers. The p53 proteins (originally thought to be, and often s ...
either for degradation (K48 polyubiquitin chain), or for nuclear export (monoubiquitylation). These events occur in a concentration dependent fashion, suggesting that modulating E3 ligase concentration is a cellular regulatory strategy for controlling protein homeostasis and localization.
Substrate recognition
Ubiquitin ligases are the final, and potentially the most important determinant of substrate specificity inubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
ation of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s. The ligases must simultaneously distinguish their protein substrate from thousands of other proteins in the cell
Cell most often refers to:
* Cell (biology), the functional basic unit of life
Cell may also refer to:
Locations
* Monastic cell, a small room, hut, or cave in which a religious recluse lives, alternatively the small precursor of a monastery ...
, and from other (ubiquitination-inactive) forms of the same protein. This can be achieved by different mechanisms, most of which involve recognition of degron
A degron is a portion of a protein that is important in regulation of protein degradation rates. Known degrons include short amino acid sequences, structural motifs and exposed amino acids (often Lysine or Arginine) located anywhere in the prote ...
s: specific short amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
sequences or chemical motifs on the substrate.
N-degrons
Proteolytic cleavage
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, ...
can lead to exposure of residues at the N-terminus of a protein. According to the N-end rule The ''N''-end rule is a rule that governs the rate of protein degradation through recognition of the N-terminal residue of proteins. The rule states that the ''N''-terminal amino acid of a protein determines its half-life (time after which half of ...
, different N-terminal amino acids (or N-degrons) are recognized to a different extent by their appropriate ubiquitin ligase (N-recognin), influencing the half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of the protein. For instance, positively charged ( Arg, Lys, His
His or HIS may refer to:
Computing
* Hightech Information System, a Hong Kong graphics card company
* Honeywell Information Systems
* Hybrid intelligent system
* Microsoft Host Integration Server
Education
* Hangzhou International School, in ...
) and bulky hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, t ...
amino acids ( Phe, Trp, Tyr, Leu, Ile
Ile may refer to:
* iLe, a Puerto Rican singer
* Ile District (disambiguation), multiple places
* Ilé-Ifẹ̀, an ancient Yoruba city in south-western Nigeria
* Interlingue (ISO 639:ile), a planned language
* Isoleucine, an amino acid
* Anothe ...
) are recognized preferentially and thus considered destabilizing degron
A degron is a portion of a protein that is important in regulation of protein degradation rates. Known degrons include short amino acid sequences, structural motifs and exposed amino acids (often Lysine or Arginine) located anywhere in the prote ...
s since they allow faster degradation of their proteins.
Phosphodegrons
 A degron can be converted into its active form by a
A degron can be converted into its active form by a post-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribos ...
such as phosphorylation of a tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the G ...
, serine or threonine residue. In this case, the ubiquitin ligase exclusively recognizes the phosphorylated version of the substrate due to stabilization within the binding site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may includ ...
. For example, FBW7, the F-box
F-box proteins are proteins containing at least one F-box domain. The first identified F-box protein is one of three components of the SCF complex, which mediates ubiquitination of proteins targeted for degradation by the 26S proteasome.
Core co ...
substrate recognition unit of an SCFFBW7ubiquitin ligase, stabilizes a phosphorylated substrate by hydrogen binding its arginine residues to the phosphate, as shown in the figure to the right. In absence of the phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
, residues of FBW7 repel the substrate.
Oxygen and small molecule dependent degrons
Presence ofoxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as ...
or other small molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
s can influence degron recognition. The von Hippel-Lindau
The term ''von'' () is used in German language surnames either as a nobiliary particle indicating a noble patrilineality, or as a simple preposition used by commoners that means ''of'' or ''from''.
Nobility directories like the ''Almanach de Go ...
(VHL) protein (substrate recognition part of a specific E3 ligase), for instance, recognizes the hypoxia-inducible factor alpha (HIF-α) only under normal oxygen conditions, when its proline is hydroxylated. Under hypoxia, on the other hand, HIF-a is not hydroxylated, evades ubiquitination
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
and thus operates in the cell at higher concentrations which can initiate transcriptional response to hypoxia. Another example of small molecule control of protein degradation is phytohormone
Plant hormone (or phytohormones) are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, from embryogenesis, the regulation of organ size, ...
auxin in plants. Auxin binds to TIR1 (the substrate recognition domain of SCFTIR1ubiquitin ligase) increasing the affinity of TIR1 for its substrates (transcriptional repressors: Aux/IAA), and promoting their degradation.
Misfolded and sugar degrons
In addition to recognizing amino acids, ubiquitin ligases can also detect unusual features on substrates that serve as signals for their destruction. For example, San1 ( Sir antagonist 1), anuclear protein A nuclear protein is a protein found in the cell nucleus. Proteins are transported inside the nucleus with the help of the nuclear pore complex, which acts a barrier between cytoplasm and nuclear membrane. The import and export of proteins through ...
quality control in yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to constit ...
, has a disordered substrate binding domain
In molecular biology, binding domain is a protein domain which binds to a specific atom or molecule, such as calcium or DNA. A protein domain is a part of a protein sequence and a tertiary structure that can change or evolve, function, and liv ...
, which allows it to bind to hydrophobic domains of misfolded proteins
Protein folding is the physical process by which a protein chain is translated to its native three-dimensional structure, typically a "folded" conformation by which the protein becomes biologically functional. Via an expeditious and reproduci ...
. Misfolded or excess unassembled glycoproteins of the ERAD
Endoplasmic-reticulum-associated protein degradation (ERAD) designates a cellular pathway which targets misfolded proteins of the endoplasmic reticulum for ubiquitination and subsequent degradation by a protein-degrading complex, called the prote ...
pathway, on the other hand, are recognized by Fbs1 and Fbs2, mammalian F-box protein
F-box proteins are proteins containing at least one F-box domain. The first identified F-box protein is one of three components of the SCF complex, which mediates ubiquitination of proteins targeted for degradation by the 26S proteasome.
Core c ...
s of E3 ligases SCFFbs1and SCFFbs2. These recognition domains have small hydrophobic pockets allowing them to bind high-mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation ...
containing glycans.
Structural motifs
In addition to lineardegron
A degron is a portion of a protein that is important in regulation of protein degradation rates. Known degrons include short amino acid sequences, structural motifs and exposed amino acids (often Lysine or Arginine) located anywhere in the prote ...
s, the E3 ligase can in some cases also recognize structural motifs on the substrate. In this case, the 3D motif can allow the substrate to directly relate its biochemical function to ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
ation. This relation can be demonstrated with TRF1 protein (regulator of human telomere
A telomere (; ) is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. Although there are different architectures, telomeres, in a broad sense, are a widespread genetic feature mos ...
length), which is recognized by its corresponding E3 ligase (FBXO4
F-box only protein 4 is a protein that in humans is encoded by the ''FBXO4'' gene.
Function
This gene encodes a member of the F-box protein family which is characterized by an approximately 40 amino acid motif, the F-box. The F-box proteins co ...
) via an intermolecular
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
beta sheet interaction. TRF1 cannot be ubiquinated while telomere bound, likely because the same TRF1 domain that binds to its E3 ligase also binds to telomeres.
Disease relevance
E3 ubiquitin ligases regulate homeostasis, cell cycle, and DNA repair pathways, and as a result, a number of these proteins are involved in a variety of cancers, including famously MDM2,BRCA1
Breast cancer type 1 susceptibility protein is a protein that in humans is encoded by the ''BRCA1'' () gene. Orthologs are common in other vertebrate species, whereas invertebrate genomes may encode a more distantly related gene. ''BRCA1'' is a ...
, and Von Hippel-Lindau tumor suppressor
The term ''von'' () is used in German language surnames either as a nobiliary particle indicating a noble patrilineality, or as a simple preposition used by commoners that means ''of'' or ''from''.
Nobility directories like the ''Almanach de ...
. For example, a mutation of MDM2 has been found in stomach cancer, renal cell carcinoma
Renal cell carcinoma (RCC) is a kidney cancer that originates in the lining of the Proximal tubule, proximal convoluted tubule, a part of the very small tubes in the kidney that transport primary urine. RCC is the most common type of kidney cance ...
, and liver cancer (amongst others) to deregulate MDM2 concentrations by increasing its promoter’s affinity for the Sp1 transcription factor
Transcription factor Sp1, also known as specificity protein 1* is a protein that in humans is encoded by the SP1 gene.
Function
The protein encoded by this gene is a zinc finger transcription factor that binds to GC-rich motifs of many promot ...
, causing increased transcription of MDM2 mRNA. Several proteomics-based experimental techniques are available for identifying E3 ubiquitin ligase-substrate pairs, such as proximity-dependent biotin identification (BioID), ubiquitin ligase-substrate trapping, and tandem ubiquitin-binding entities (TUBEs).
Examples
* ARING
Ring may refer to:
* Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry
* To make a sound with a bell, and the sound made by a bell
:(hence) to initiate a telephone connection
Arts, entertainment and media Film and ...
(''R''eally ''I''nteresting ''N''ew ''G''ene) domain binds the E2 conjugase and might be found to mediate enzymatic activity in the E2-E3 complex
* An F-box domain (as in the SCF complex) binds the ubiquitinated substrate. (e.g., Cdc 4, which binds the target protein Sic1
Sic1, a protein, is a stoichiometric inhibitor of Cdk1-Clb ( B-type cyclins) complexes in the budding yeast ''Saccharomyces cerevisiae''. Because B-type cyclin-Cdk1 complexes are the drivers of S-phase initiation, Sic1 prevents premature S-phase ...
; Grr1, which binds Cln).
* A HECT domain
In molecular biology, the HECT domain is a protein domain found in ubiquitin-protein ligases. The name HECT comes from 'Homologous to the E6-AP Carboxyl Terminus'. Proteins containing this domain at the C terminus include ubiquitin-protein ligase ...
, which is involved in the transfer of ubiquitin from the E2 to the substrate.
Individual E3 ubiquitin ligases
* E3A * mdm2 *Anaphase-promoting complex
Anaphase-promoting complex (also called the cyclosome or APC/C) is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cull ...
(APC)
* UBR5
E3 ubiquitin-protein ligase UBR5 is an enzyme that in humans is encoded by the ''UBR5'' gene.
Function
This gene encodes a progestin-induced protein, which belongs to the HECT (homology to E6-AP carboxyl terminus) family. The HECT family protei ...
(EDD1)
* SOCS
SOCS (suppressor of cytokine signaling proteins) refers to a family of genes involved in inhibiting the JAK-STAT signaling pathway.
Genes
* CISH
* SOCS1
* SOCS2
* SOCS3
* SOCS4
* SOCS5
* SOCS6
* SOCS7
Suppressor of cytokine signaling 7 is a pro ...
/ BC-box/ eloBC/ CUL5
Cullin-5 is a protein that in humans is encoded by the ''CUL5'' gene.
Discovery
The mammalian gene product was originally discovered by expression cloning, due to the protein's ability to mobilize intracellular calcium in response to the peptide ...
/ RING
Ring may refer to:
* Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry
* To make a sound with a bell, and the sound made by a bell
:(hence) to initiate a telephone connection
Arts, entertainment and media Film and ...
* LNXp80
* CBX4, CBLL1
* HACE1
HECT domain and ankyrin repeat containing E3 ubiquitin protein ligase 1 is a protein that in humans is encoded by the HACE1 gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian uni ...
* HECTD1, HECTD2, HECTD3, HECTD4
* HECW1, HECW2
* HERC1, HERC2 HERC2 is a giant E3 ubiquitin protein ligase, implicated in DNA repair regulation, pigmentation and neurological disorders. It is encoded by a gene of the same name belonging to the HERC family, which typically encodes large protein products with C ...
, HERC3, HERC4, HERC5
Probable E3 ubiquitin-protein ligase HERC5 is an enzyme that in humans is encoded by the ''HERC5'' gene.
This gene is a member of the HERC family of ubiquitin ligases and encodes a protein with a HECT domain and five RCC1 repeats. Pro-inflammator ...
, HERC6
* HUWE1
E3 ubiquitin-protein ligase HUWE1 is an enzyme that in humans is encoded by the ''HUWE1'' gene.
It performs the third step (ligation) in binding ubiquitin to proteins in a process called ubiquitination which tags the proteins for disposal.
Human ...
, ITCH
Itch (also known as pruritus) is a sensation that causes the desire or reflex to scratch. Itch has resisted many attempts to be classified as any one type of sensory experience. Itch has many similarities to pain, and while both are unpleasant ...
* NEDD4
E3 ubiquitin-protein ligase NEDD4, also known as neural precursor cell expressed developmentally down-regulated protein 4 (whence "NEDD4") is an enzyme that is, in humans, encoded by the ''NEDD4'' gene.
NEDD4 is an E3 ubiquitin ligase enzyme, that ...
, NEDD4L
Neural precursor cell expressed developmentally downregulated gene 4-like (NEDD4L) or NEDD4-2 is an enzyme (ubiquitin ligase) of the NEDD4 family.
In human the protein is encoded by the ''NEDD4L'' gene. In mouse the protein is commonly known as NE ...
* PPIL2
Peptidyl-prolyl cis-trans isomerase-like 2 is an enzyme that in humans is encoded by the ''PPIL2'' gene.
This gene is a member of the cyclophilin family of peptidylprolyl isomerases. The cyclophilins are a highly conserved ubiquitous family, membe ...
* PRPF19
* PIAS1
E3 SUMO-protein ligase PIAS1 is an enzyme that in humans is encoded by the ''PIAS1'' gene.
Function
This gene encodes a member of the mammalian PIAS rotein inhibitor of activated STAT-1 (signal transducer and activator of transcription-1)famil ...
, PIAS2
E3 SUMO-protein ligase PIAS2 is an enzyme that in humans is encoded by the ''PIAS2'' gene.
Interactions
Protein inhibitor of activated STAT2 has been shown to interact with:
* Androgen receptor,
* DNMT3A,
* PARK7, and
* UBE2I
SUMO-conjugat ...
, PIAS3
E3 SUMO-protein ligase PIAS3 is an enzyme that in humans is encoded by the PIAS3 gene.
PIAS family
The mammalian PIAS family consists of four members: PIAS1, PIAS2, PIAS3 and PIAS4. In Drosophila, a single PIAS homologue named dPIAS/Zimp has be ...
, PIAS4
E3 SUMO-protein ligase PIAS4 is one of several protein inhibitor of activated STAT (PIAS) proteins. It is also known as protein inhibitor of activated STAT protein gamma (PIASg or PIASy), and is an enzyme that in humans is encoded by the ''PIAS4' ...
* RANBP2
RAN binding protein 2 (RANBP2) is protein which in humans is encoded by the ''RANBP2'' gene. It is also known as nucleoporin 358 (Nup358) since it is a member nucleoporin family that makes up the nuclear pore complex. RanBP2 has a mass of 358 kDa. ...
* RNF4
* RBX1
RING-box protein 1 is a protein that in humans is encoded by the ''RBX1'' gene.
Function
This gene encodes an evolutionarily conserved protein that interacts with cullins. The protein plays a unique role in the ubiquitination reaction by heter ...
* SMURF1
E3 ubiquitin-protein ligase SMURF1 is an enzyme that in humans is encoded by the ''SMURF1'' gene.
Function
This gene encodes a ubiquitin ligase that is specific for receptor-regulated SMAD proteins in the bone morphogenetic protein ( BMP) pat ...
, SMURF2
E3 ubiquitin-protein ligase SMURF2 is an enzyme that in humans is encoded by the ''SMURF2'' gene which is located at chromosome 17q23.3-q24.1.
Interactions
SMURF2 has been shown to interact with:
* Mothers against decapentaplegic homolog 1,
* ...
* STUB1
STUB1 (STIP1 homology and U-Box containing protein 1) is a human gene that codes for the protein CHIP (C terminus of HSC70-Interacting Protein).
Function
The CHIP protein encoded by this gene binds to and inhibits the ATPase activity of the cha ...
* TOPORS
* TRIP12
Probable E3 ubiquitin-protein ligase TRIP12 is an enzyme that in humans is encoded by the ''TRIP12'' gene.
Interactions
TRIP12 has been shown to interact with APPBP1
NEDD8-activating enzyme E1 regulatory subunit is a protein that in humans i ...
* UBE3A
Ubiquitin-protein ligase E3A (UBE3A) also known as E6AP ubiquitin-protein ligase (E6AP) is an enzyme that in humans is encoded by the ''UBE3A'' gene. This enzyme is involved in targeting proteins for degradation within cells.
Protein degradation ...
, UBE3B, UBE3C, UBE3D
* UBE4A, UBE4B
* UBOX5
* UBR5
E3 ubiquitin-protein ligase UBR5 is an enzyme that in humans is encoded by the ''UBR5'' gene.
Function
This gene encodes a progestin-induced protein, which belongs to the HECT (homology to E6-AP carboxyl terminus) family. The HECT family protei ...
* VHL
* WWP1
NEDD4-like E3 ubiquitin-protein ligase WWP1 is an enzyme that in humans is encoded by the ''WWP1'' gene.
Function
WW domain-containing proteins are found in all eukaryotes and play an important role in the regulation of a wide variety of cellul ...
, WWP2
NEDD4-like E3 ubiquitin-protein ligase WWP2 also known as atrophin-1-interacting protein 2 (AIP2) or WW domain-containing protein 2 (WWP2) is an enzyme that in humans is encoded by the ''WWP2'' gene.
Function
This gene encodes a member of the ...
* Parkin
* MKRN1
See also
*ERAD
Endoplasmic-reticulum-associated protein degradation (ERAD) designates a cellular pathway which targets misfolded proteins of the endoplasmic reticulum for ubiquitination and subsequent degradation by a protein-degrading complex, called the prote ...
* Ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
* Ubiquitin-activating enzyme
Ubiquitin-activating enzymes, also known as E1 enzymes, catalyze the first step in the ubiquitination reaction, which (among other things) can target a protein for degradation via a proteasome. This covalent bond of ubiquitin or ubiquitin-like pro ...
* Ubiquitin-conjugating enzyme
Ubiquitin-conjugating enzymes, also known as E2 enzymes and more rarely as ''ubiquitin-carrier enzymes'', perform the second step in the ubiquitination reaction that targets a protein for degradation via the proteasome. The ubiquitination process ...
References
External links
Quips article describing E3 Ligase function
a
PDBe
* * {{DEFAULTSORT:Ubiquitin Ligase EC 6.3 Post-translational modification