UV degradation on:
[Wikipedia]
[Google]
[Amazon]

 In
In
 Susceptibility to photo-oxidation varies depending on the chemical structure of the polymer. Some materials have excellent stability, such as
Susceptibility to photo-oxidation varies depending on the chemical structure of the polymer. Some materials have excellent stability, such as
 Chain initiation
::
Chain initiation
:: Polymer -> P\bullet +\ P\bullet
Chain propagation
::P\bullet +\ O2 -> POO\bullet
::POO\bullet +\ PH -> +\ P\bullet
Chain branching
::POOH -> PO\bullet +\ OH\bullet
:: + OH\bullet -> P\bullet +\ H2O
::PO\bullet -> Chain\ scission\ reactions
Termination
::POO\bullet +\ POO\bullet -> cross\ linking\ reaction\ to\ non-radical\ product
::POO\bullet +\ P\bullet -> cross\ linking\ reaction\ to\ non-radical\ product
:: P\bullet +\ P\bullet -> cross\ linking\ reaction\ to\ non-radical\ product
Classically the carbon-centred macroradicals (P•) rapidly react with oxygen to form hydroperoxyl radicals (POO•), which in turn abstract an H atom from the polymer chain to give a hydroperoxide (POOH) and a fresh macroradical. Hydroperoxides readily undergo  Secondary hydroperoxides can also undergo an intramolecular reaction to give a ketone group, although this is limited to polyethylene.
:
Secondary hydroperoxides can also undergo an intramolecular reaction to give a ketone group, although this is limited to polyethylene.
: The ketones generated by these processes are themselves photo-active, although much more weakly. At ambient temperatures they undergo Type II Norrish reactions with chain scission. They may also absorb UV-energy, which they can then transfer to O2, causing it to enter its highly reactive
The ketones generated by these processes are themselves photo-active, although much more weakly. At ambient temperatures they undergo Type II Norrish reactions with chain scission. They may also absorb UV-energy, which they can then transfer to O2, causing it to enter its highly reactive 
 For
For  Polystyrene is observed to yellow during photo-oxidation, which is attributed to the formation of
Polystyrene is observed to yellow during photo-oxidation, which is attributed to the formation of
 When the polyenes contain at least eight conjugated double bonds they become coloured, leading to yellowing and eventual browning of the material. This is off-set slightly by longer polyenes being photobleached with atmospheric oxygen, however PVC does eventually discolour unless polymer stabilisers are present. Reactions at organochloride sites proceed via the usual hydroperoxyl and hydroperoxide before photolysis yields the α-chloro-alkoxyl radical. This species can undergo various reactions to give carbonyls, peroxide
When the polyenes contain at least eight conjugated double bonds they become coloured, leading to yellowing and eventual browning of the material. This is off-set slightly by longer polyenes being photobleached with atmospheric oxygen, however PVC does eventually discolour unless polymer stabilisers are present. Reactions at organochloride sites proceed via the usual hydroperoxyl and hydroperoxide before photolysis yields the α-chloro-alkoxyl radical. This species can undergo various reactions to give carbonyls, peroxide 
 Type II Norrish reactions are less common but give rise to
Type II Norrish reactions are less common but give rise to  Radicals formed by photolysis may initiate the photo-oxidation in PET. Photo-oxidation of the aromatic
Radicals formed by photolysis may initiate the photo-oxidation in PET. Photo-oxidation of the aromatic 
EU directive 5 June 2019
 The photo-oxidation of polymers can be investigated by either natural or accelerated weather testing. Such testing is important in determining the expected service-life of plastic items as well as the fate of waste plastic.
The photo-oxidation of polymers can be investigated by either natural or accelerated weather testing. Such testing is important in determining the expected service-life of plastic items as well as the fate of waste plastic.
 Degradation can be detected before serious cracks are seen in a product by using
Degradation can be detected before serious cracks are seen in a product by using

 In
In polymer chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry ar ...
photo-oxidation (sometimes: oxidative photodegradation Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroy ...
) is the degradation of a polymer surface due to the combined action of light and oxygen. It is the most significant factor in the weathering of plastics. Photo-oxidation causes the polymer chains to break (chain scission
Chain scission is a term used in polymer chemistry describing the degradation of a polymer main chain. It is often caused by thermal stress (heat) or ionizing radiation (e.g. light, UV radiation or gamma radiation), often involving oxygen. Durin ...
), resulting in the material becoming increasingly brittle. This leads to mechanical failure
Structural integrity and failure is an aspect of engineering that deals with the ability of a structure to support a designed structural load (weight, force, etc.) without breaking and includes the study of past structural failures in order to ...
and, at an advanced stage, the formation of microplastics
Microplastics are fragments of any type of plastic less than in length, according to the U.S. National Oceanic and Atmospheric Administration (NOAA) and the European Chemicals Agency. They cause pollution by entering natural ecosystems from a ...
. In textile
Textile is an umbrella term that includes various fiber-based materials, including fibers, yarns, filaments, threads, different fabric types, etc. At first, the word "textiles" only referred to woven fabrics. However, weaving is not the ...
s the process is called phototendering Phototendering is the process by which organic fibres and textiles lose strength and flexibility as a result of exposure to sunlight. It is the ultraviolet component of the sun's spectrum which affects fibres, causing chain degradation and hence l ...
.
Technologies have been developed to both accelerate and inhibit this process. For example, plastic building components like doors, window frames and gutters are expected to last for decades, requiring the use of advanced UV-polymer stabilizers Polymer stabilizers (British: polymer stabilisers) are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation.
Common polymer degradation processes include oxidation, UV-d ...
. Conversely, single-use plastics can be treated with biodegradable additives
Biodegradable additives are additives that enhance the biodegradation of polymers by allowing microorganisms to utilize the carbon within the polymer chain as a source of energy. Biodegradable additives attract microorganisms to the polymer through ...
to accelerate their fragmentation.
Many pigments
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
and dyes
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
can similarly have effects due to their ability to absorb UV-energy.
Susceptible polymers
fluoropolymers
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon," ...
, polyimides
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. ...
, silicone
A silicone or polysiloxane is a polymer made up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking ...
s and certain acrylate polymer
An acrylate polymer (also known as acrylic or polyacrylate) is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.
Acrylate polymer is commonly used ...
s. However, global polymer production is dominated by a range of commodity plastics
Commodity plastics or commodity polymers are plastics produced in high volumes for applications where exceptional material properties are not needed (such as packaging, food containers, and household products). In contrast to engineering plastics ...
which account for the majority of plastic waste
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are categ ...
. Of these polyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, ...
(PET) has only moderate UV resistance and the others, which include polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a ...
, polyvinyl chloride (PVC) and polyolefins
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
like polypropylene (PP) and polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
(PE) are all highly susceptible.
Photo-oxidation is a form of photodegradation Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroy ...
and begins with formation of free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
s on the polymer chain, which go on to react with oxygen in chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
s. For many polymers the general autoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organi ...
mechanism is a reasonable approximation of the underlying chemistry. The process is autocatalytic
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 199 ...
, generating increasing numbers of radicals and reactive oxygen species. These reactions result in changes to the molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
(and molecular weight distribution The molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer species (Ni) and the molar mass (Mi) of that species. In linear polymers, the individual polymer chains rarely hav ...
) of the polymer and as a consequence of this the material becomes more brittle. The general process can be divided into four stages:
:Initiation the process of generating the initial free radical.
:Propagation the conversion of one active species to another
:Chain branching steps which end with more than one active species being produced. The photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
of hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. ...
s is the main example.
:Termination steps in which active species are removed, for instance by radical disproportionation Radical disproportionation encompasses a group of reactions in organic chemistry in which two radicals react to form two different non-radical products. Radicals in chemistry are defined as reactive atoms or molecules that contain an unpaired elect ...
Photo-oxidation can occur simultaneously with other processes like thermal degradation
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
, and each of these can accelerate the other.
Polyolefins
Polyolefins
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
such as polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
and polypropylene are susceptible to photo-oxidation and around 70% of light stabilizers produced world-wide are used in their protection, despite them representing only around 50% of global plastic production. Aliphatic hydrocarbons can only adsorb high energy UV-rays with a wavelength below ~250nm, however the Earth’s atmosphere and ozone layer
The ozone layer or ozone shield is a region of Earth's stratosphere that absorbs most of the Sun's ultraviolet radiation. It contains a high concentration of ozone (O3) in relation to other parts of the atmosphere, although still small in relat ...
screen out such rays, with the normal minimum wavelength being 280-290 nm.
The bulk of the polymer is therefore photo-inert and degradation is instead attributed to the presence of various impurities, which are introduced during the manufacturing or processing stages. These include hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. ...
and carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
groups, as well as metal salts such as catalyst residues.
All of these species act as photoinitiator
A photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (UV or visible). Synthetic photoinitiators are key components in photopolymers (for example, photo-curable coatings, adhesive ...
s.
The organic groups are able to absorb UV light above 290 nm whereupon they undergo photolysis to generate radicals. Metal impurities act as photocatalysts, although such reactions can be complex. It has also been suggested that polymer-O2 charge-transfer complex
In chemistry, a charge-transfer (CT) complex or electron-donor-acceptor complex describes a type of supramolecular assembly of two or more molecules or ions. The assembly consists of two molecules that self-attract through electrostatic forces, ...
es are involved. Initiation generates radical-carbons on the polymer chain, sometimes called macroradicals (P•).
 Chain initiation
::
Chain initiation
:: photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
to give an alkoxyl macroradical radical (PO•) and a hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
(HO•), both of which may go on to form new polymer radicals via hydrogen abstraction. Non-classical alternatives to these steps have been proposed. The alkoxyl radical may also undergo beta scission
Beta scission is an important reaction in the chemistry of thermal cracking of hydrocarbons and the formation of free radicals. Free radicals are formed upon splitting the carbon-carbon bond. Free radicals are extremely reactive and short-lived. ...
, generating a acyl-ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
and macroradical. This is considered to be the main cause of chain breaking in polypropylene.
: Secondary hydroperoxides can also undergo an intramolecular reaction to give a ketone group, although this is limited to polyethylene.
:
Secondary hydroperoxides can also undergo an intramolecular reaction to give a ketone group, although this is limited to polyethylene.
: The ketones generated by these processes are themselves photo-active, although much more weakly. At ambient temperatures they undergo Type II Norrish reactions with chain scission. They may also absorb UV-energy, which they can then transfer to O2, causing it to enter its highly reactive
The ketones generated by these processes are themselves photo-active, although much more weakly. At ambient temperatures they undergo Type II Norrish reactions with chain scission. They may also absorb UV-energy, which they can then transfer to O2, causing it to enter its highly reactive triplet state
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1.
Spin, in the context of quantum mechanics, is not a mechanical ...
. Triplet oxygen is a potent oxidising agent can go on to form cause further degradation.
:Polystyrene
 For
For polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a ...
the complete mechanism of photo-oxidation is still a matter of debate, as different pathways may operate concurrently and vary according to the wavelength of the incident light.
Regardless, there is agreement on the major steps.
Pure polystyrene should not be able to absorb light with a wavelength below ~280nm and initiation is explained though photo-labile impurities (hydroperoxides) and charge transfer complexes, all of which are able to absorb normal sunlight. Charge-transfer complex
In chemistry, a charge-transfer (CT) complex or electron-donor-acceptor complex describes a type of supramolecular assembly of two or more molecules or ions. The assembly consists of two molecules that self-attract through electrostatic forces, ...
es of oxygen and polystyrene phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydroge ...
s absorb light to form singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
, which acts as a radical initiator. Carbonyl impurities in the polymer (c.f. acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene p ...
) also absorb light in the near ultraviolet range (300 to 400nm), forming excited ketones able to abstract hydrogen atoms directly from the polymer. Hyroperoxide undergoes photolysis to form hydroxyl and alkoxyl radicals.
These initiation steps generate macroradicals at tertiary sites, as these are more stabilised. The propagation steps are essentially identical to those seen for polyolefins; with oxidation, hydrogen abstraction
In chemistry, a hydrogen atom abstraction or hydrogen atom transfer (HAT) is any chemical reaction in which a hydrogen free radical (neutral hydrogen atom) is abstracted from a substrate according to the general equation:
:X^\bullet + H-Y -> X-H ...
and photolysis leading to beta scission
Beta scission is an important reaction in the chemistry of thermal cracking of hydrocarbons and the formation of free radicals. Free radicals are formed upon splitting the carbon-carbon bond. Free radicals are extremely reactive and short-lived. ...
reactions and increasing numbers of radicals.
These steps account for the majority of chain-breaking, however in a minor pathway the hydroperoxide reacts directly with polymer to form a ketone group (acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene p ...
) and a terminal alkene without the formation of additional radicals.
: Polystyrene is observed to yellow during photo-oxidation, which is attributed to the formation of
Polystyrene is observed to yellow during photo-oxidation, which is attributed to the formation of polyene
In organic chemistry, polyenes are poly- unsaturated, organic compounds that contain at least three alternating double () and single () carbon–carbon bonds. These carbon–carbon double bonds interact in a process known as conjugation, result ...
s from these terminal alkenes.
Polyvinyl chloride - (PVC)
Pureorganochloride
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlor ...
s like polyvinyl chloride (PVC) do not absorb any light above 220 nm. The initiation of photo-oxidation is instead caused by various irregularities in the polymer chain, such as structural defects as well as hydroperoxides, carbonyl groups, and double bonds.
Hydroperoxides formed during processing are the most important initiator to begin with, however their concentration decreases during photo-oxidation whereas carbonyl concentration increases, as such carbonyls may become the primary initiator over time.
Propagation steps involve the hydroperoxyl radical, which can abstract hydrogen from both hydrocarbon (-CH2-) and organochloride (-CH2Cl-) sites in the polymer at comparable rates. Radicals formed at hydrocarbon sites rapidly convert to alkenes with loss of radical chlorine. This forms allylic
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . ...
hydrogens (shown in red) which are more susceptible to hydrogen abstraction leading to the formation of polyene
In organic chemistry, polyenes are poly- unsaturated, organic compounds that contain at least three alternating double () and single () carbon–carbon bonds. These carbon–carbon double bonds interact in a process known as conjugation, result ...
s in zipper-like reactions.
: When the polyenes contain at least eight conjugated double bonds they become coloured, leading to yellowing and eventual browning of the material. This is off-set slightly by longer polyenes being photobleached with atmospheric oxygen, however PVC does eventually discolour unless polymer stabilisers are present. Reactions at organochloride sites proceed via the usual hydroperoxyl and hydroperoxide before photolysis yields the α-chloro-alkoxyl radical. This species can undergo various reactions to give carbonyls, peroxide
When the polyenes contain at least eight conjugated double bonds they become coloured, leading to yellowing and eventual browning of the material. This is off-set slightly by longer polyenes being photobleached with atmospheric oxygen, however PVC does eventually discolour unless polymer stabilisers are present. Reactions at organochloride sites proceed via the usual hydroperoxyl and hydroperoxide before photolysis yields the α-chloro-alkoxyl radical. This species can undergo various reactions to give carbonyls, peroxide cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
s and beta scission products.

Poly(ethylene terephthalate) - (PET)
Unlike most other commodity plasticspolyethylene terephthalate
Polyethylene terephthalate (or poly(ethylene terephthalate), PET, PETE, or the obsolete PETP or PET-P), is the most common thermoplastic polymer resin of the polyester family and is used in fibres for clothing, containers for liquids and foods, ...
(PET) is able to absorb the near ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation i ...
rays in sunlight. Absorption begins at 360 nm, becoming stronger below 320 nm and is very significant below 300 nm. Despite this PET has better resistance to photo-oxidation than other commodity plastics
Commodity plastics or commodity polymers are plastics produced in high volumes for applications where exceptional material properties are not needed (such as packaging, food containers, and household products). In contrast to engineering plastics ...
, this is due to a poor quantum yield The quantum yield (Φ) of a radiation-induced process is the number of times a specific event occurs per photon absorbed by the system.
Applications
Fluorescence spectroscopy
The fluorescence quantum yield is defined as the ratio of the numb ...
or the absorption. The degradation chemistry is complicated due to simultaneous photodissociation
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
(i.e. not involving oxygen) and photo-oxidation reactions of both the aromatic and aliphatic parts of the molecule. Chain scission is the dominate process, with chain branching and the formation of coloured impurities being less common. Carbon monoxide, carbon dioxide, and carboxylic acids are the main products.
The photo-oxidation of other linear polyesters such as polybutylene terephthalate
Polybutylene terephthalate (PBT) is a thermoplastic engineering polymer that is used as an insulator in the electrical and electronics industries. It is a thermoplastic (semi-)crystalline polymer, and a type of polyester. PBT resists solvents, s ...
and polyethylene naphthalate
Polyethylene naphthalate (poly(ethylene 2,6-naphthalate) or PEN) is a polyester derived from naphthalene-2,6-dicarboxylic acid and ethylene glycol. As such it is related to poly(ethylene terephthalate), but with superior barrier properties.
Prod ...
proceeds similarly.
Photodissociation involves the formation of an excited terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced ann ...
unit which undergoes Norrish reactions. The type I reaction dominates, which cause chain scission at the carbonyl unit to give a range of products.
 Type II Norrish reactions are less common but give rise to
Type II Norrish reactions are less common but give rise to acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the m ...
by way of vinyl alcohol esters. This has an exceedingly low odour and taste threshold and can cause an off-taste in bottled water.
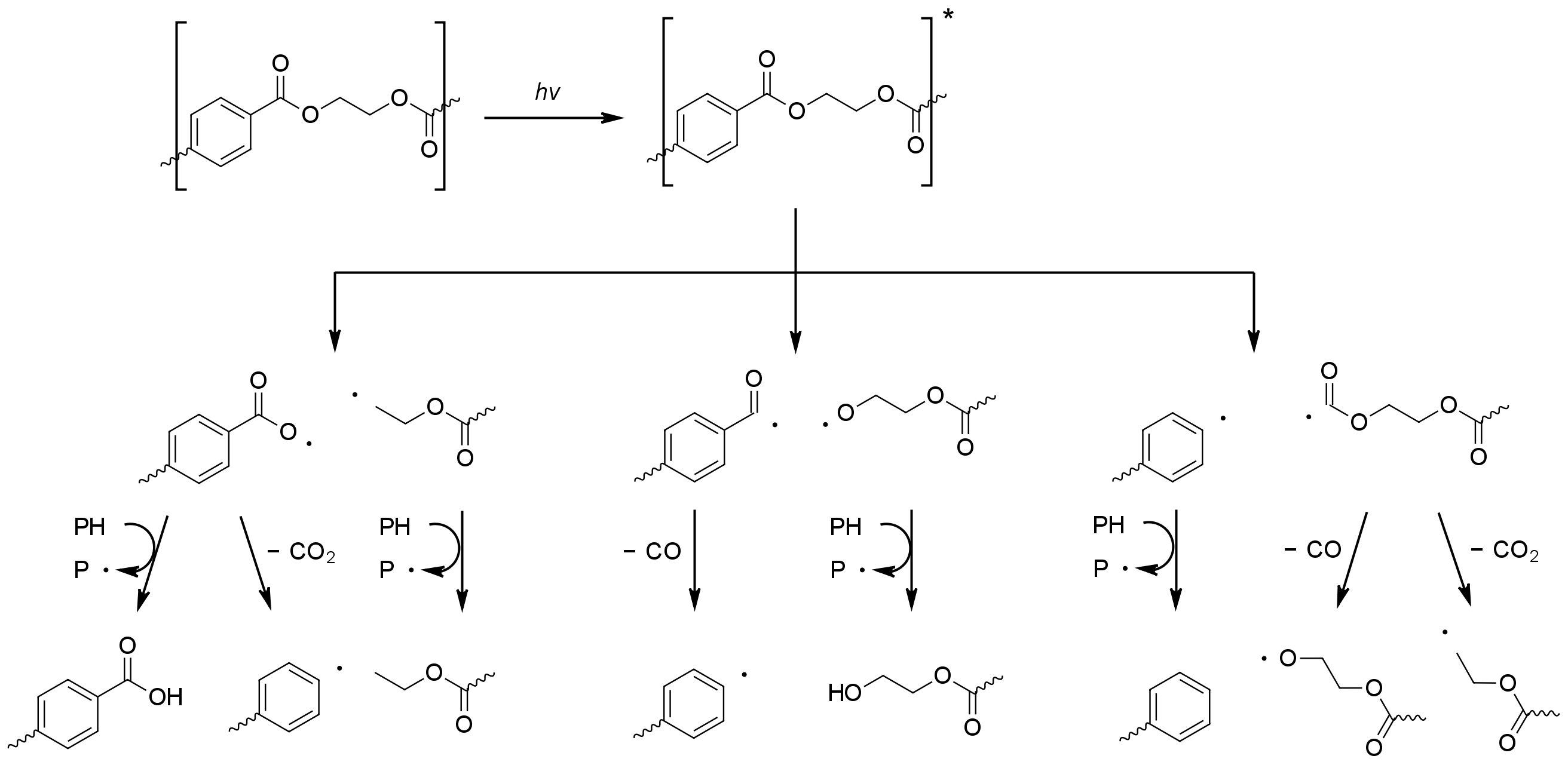
 Radicals formed by photolysis may initiate the photo-oxidation in PET. Photo-oxidation of the aromatic
Radicals formed by photolysis may initiate the photo-oxidation in PET. Photo-oxidation of the aromatic terephthalic acid
Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tonnes are produced ann ...
core results in its step-wise oxidation to 2,5-dihydroxyterephthalic acid. The photo-oxidation process at aliphatic sites is similar to that seen for polyolefins, with the formation of hydroperoxide species eventually leading to beta-scission of the polymer chain.

Secondary factors
Environment
Perhaps surprisingly, the effect of temperature is often greater than the effect of UV exposure. This can be seen in terms of theArrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 18 ...
, which shows that reaction rates have an exponential dependence on temperature. By comparison the dependence of degradation rate on UV exposure and the availability of oxygen is broadly linear. As the oceans are cooler than land plastic pollution
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are catego ...
in the marine environment degrades more slowly. Materials buried in landfill
A landfill site, also known as a tip, dump, rubbish dump, garbage dump, or dumping ground, is a site for the disposal of waste materials. Landfill is the oldest and most common form of waste disposal, although the systematic burial of the waste ...
do not degrade by photo-oxidation at all, though they may gradually decay by other processes.
Mechanical stress
In continuum mechanics, stress is a physical quantity. It is a quantity that describes the magnitude of forces that cause deformation. Stress is defined as ''force per unit area''. When an object is pulled apart by a force it will cause elonga ...
can effect the rate of photo-oxidation and may also accelerate the physical breakup of plastic objects. Stress can be caused by mechanical load (tensile and shear stresses) or even by temperature cycling Temperature cycling (or temperature cycle) is the process of cycling through two temperature extremes, typically at relatively high rates of change. It is an environmental stress test used in evaluating product reliability as well as in manufacturi ...
, particularly in composite systems consisting of materials with differing temperature coefficient
A temperature coefficient describes the relative change of a physical property that is associated with a given change in temperature. For a property ''R'' that changes when the temperature changes by ''dT'', the temperature coefficient α is def ...
s of expansion. Similarly, sudden rainfall can cause thermal stress
In mechanics and thermodynamics, thermal stress is mechanical stress created by any change in temperature of a material. These stresses can lead to fracturing or plastic deformation depending on the other variables of heating, which include mat ...
.
Effects of dyes and other additives
Dye
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and ...
s and pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
s are used in polymer materials to provide colour, however they can also effect the rate of photo-oxidation. Many absorb UV rays and in so doing protect the polymer, however absorption can cause the dyes to enter an excited state where they may attack the polymer or transfer energy to O2 to form damaging singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
. Cu-phthalocyanine is an example, it strongly absorbs UV light however the excited Cu-phthalocyanine may act as a photoinitiator
A photoinitiator is a molecule that creates reactive species (free radicals, cations or anions) when exposed to radiation (UV or visible). Synthetic photoinitiators are key components in photopolymers (for example, photo-curable coatings, adhesive ...
by abstracting hydrogen atoms from the polymer. Its interactions may become even more complicated when other additives are present.
Fillers
In processed animal foods, a filler is an ingredient added to provide dietary fiber, bulk or some other non-nutritive purpose.
Products like corncobs, feathers, soy, cottonseed hulls, peanut hulls, citrus pulp, screening, weeds, straw, and cere ...
such as carbon black
Carbon black (subtypes are acetylene black, channel black, furnace black, lamp black and thermal black) is a material produced by the incomplete combustion of coal and coal tar, vegetable matter, or petroleum products, including fuel oil, flui ...
can screen out UV light, effectively stabilisers the polymer, whereas flame retardant
The term flame retardants subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source an ...
s tend to cause increased levels of photo-oxidation.
Additives to enhance degradation
Biodegradable additives
Biodegradable additives are additives that enhance the biodegradation of polymers by allowing microorganisms to utilize the carbon within the polymer chain as a source of energy. Biodegradable additives attract microorganisms to the polymer through ...
may be added to polymers to accelerate their degradation. In the case of photo-oxidation OXO-biodegradation additives are used. These are transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
salts such as iron (Fe), manganese (Mn), and cobalt (Co). Fe complexes increase the rate of photooxidation by promoting the homolysis of hydroperoxides via Fenton reaction
Fenton's reagent is a solution of hydrogen peroxide (H2O2) with ferrous iron (typically iron(II) sulfate, FeSO4) as a catalyst that is used to oxidize contaminants or waste waters as part of an advanced oxidation process. Fenton's reagent can be u ...
s.
The use of such additives has been controversial due to concerns that treated plastics do not fully biodegrade and instead result in the accelerated formation of microplastics
Microplastics are fragments of any type of plastic less than in length, according to the U.S. National Oceanic and Atmospheric Administration (NOAA) and the European Chemicals Agency. They cause pollution by entering natural ecosystems from a ...
. Oxo-plastics would be difficult to distinguish from untreated plastic but their inclusion during plastic recycling
Plastic recycling is the reprocessing of plastic waste into new products. When performed correctly, this can reduce dependence on landfill, conserve resources and protect the environment from plastic pollution and greenhouse gas emissions.
A ...
can create a destabilised product with fewer potential uses, potentially jeopardising the business case for recycling any plastic. OXO-biodegradation additives were banned in the EU in 2019the EU directive 2019/904 (Article 5)EU directive 5 June 2019
Prevention
UV attack by sunlight can be ameliorated or prevented by adding anti-UVpolymer stabilizers Polymer stabilizers (British: polymer stabilisers) are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation.
Common polymer degradation processes include oxidation, UV-d ...
, usually prior to shaping the product by injection moulding
Injection moulding (U.S. spelling: injection molding) is a manufacturing process for producing parts by injecting molten material into a mould, or mold. Injection moulding can be performed with a host of materials mainly including metals (for ...
. UV stabilizers in plastics usually act by absorbing the UV radiation preferentially, and dissipating the energy as low-level heat. The chemicals used are similar to those in sunscreen
Sunscreen, also known as sunblock or sun cream, is a photoprotective topical product for the skin that mainly absorbs, or to a much lesser extent reflects, some of the sun's ultraviolet (UV) radiation and thus helps protect against sunburn an ...
products, which protect skin from UV attack. They are used frequently in plastics
Plastics are a wide range of synthetic polymers, synthetic or semi-synthetic materials that use polymers as a main ingredient. Their Plasticity (physics), plasticity makes it possible for plastics to be Injection moulding, moulded, Extrusion, e ...
, including cosmetics
Cosmetics are constituted mixtures of chemical compounds derived from either natural sources, or synthetically created ones. Cosmetics have various purposes. Those designed for personal care and skin care can be used to cleanse or protect ...
and films
A film also called a movie, motion picture, moving picture, picture, photoplay or (slang) flick is a work of visual art that simulates experiences and otherwise communicates ideas, stories, perceptions, feelings, beauty, or atmosphere ...
. Different UV stabilizers are utilized depending upon the substrate, intended functional life, and sensitivity to UV degradation. UV stabilizers, such as benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylket ...
s, work by absorbing the UV radiation and preventing the formation of free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
s. Depending upon substitution, the UV absorption spectrum
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating fi ...
is changed to match the application. Concentrations normally range from 0.05% to 2%, with some applications up to 5%.
Frequently, glass can be a better alternative to polymers when it comes to UV degradation. Most of the commonly used glass
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) o ...
types are highly resistant to UV radiation. Explosion protection lamps for oil rigs for example can be made either from polymer or glass. Here, the UV radiation and rough weathers belabor the polymer so much, that the material has to be replaced frequently.
Poly(ethylene-naphthalate) (PEN) can be protected by applying a zinc oxide coating, which acts as protective film reducing the diffusion of oxygen. Zinc oxide can also be used on polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
(PC) to decrease the oxidation and photo-yellowing rate caused by solar radiation.
Analysis
Weather testing of polymers
 The photo-oxidation of polymers can be investigated by either natural or accelerated weather testing. Such testing is important in determining the expected service-life of plastic items as well as the fate of waste plastic.
The photo-oxidation of polymers can be investigated by either natural or accelerated weather testing. Such testing is important in determining the expected service-life of plastic items as well as the fate of waste plastic.
Detection
 Degradation can be detected before serious cracks are seen in a product by using
Degradation can be detected before serious cracks are seen in a product by using infrared spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or function ...
, which is able to detect chemical species formed by photo-oxidation. In particular, peroxy-species and carbonyl group
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
s have distinct absorption bands.
In the example shown at left, carbonyl groups were easily detected by IR spectroscopy from a cast thin film. The product was a road cone made by rotational moulding
Rotational molding ( BrE: moulding) involves a heated mold which is filled with a charge or shot weight of material. It is then slowly rotated (usually around two perpendicular axes), causing the softened material to disperse and stick to the ...
in LDPE
Low-density polyethylene (LDPE) is a thermoplastic made from the monomer ethylene. It was the first grade of polyethylene, produced in 1933 by Imperial Chemical Industries (ICI) using a high pressure process via free radical polymerization. Its ...
, which had cracked prematurely in service. Many similar cones also failed because an anti-UV additive had not been used during processing. Other plastic products which failed included polypropylene mancabs used at roadworks which cracked after service of only a few months.
See also
*Forensic polymer engineering
Forensic polymer engineering is the study of failure in polymeric products. The topic includes the fracture of plastic products, or any other reason why such a product fails in service, or fails to meet its specification. The subject focuses on t ...
*Photodegradation Photodegradation is the alteration of materials by light. Commonly, the term is used loosely to refer to the combined action of sunlight and air, which cause oxidation and hydrolysis. Often photodegradation is intentionally avoided, since it destroy ...
*Polymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle ...
*Stress corrosion cracking
Stress corrosion cracking (SCC) is the growth of crack formation in a corrosive environment. It can lead to unexpected and sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. SCC ...
*Thermal degradation of polymers In polymers, such as plastics, thermal degradation refers to a type of polymer degradation where damaging chemical changes take place at elevated temperatures, without the simultaneous involvement of other compounds such as oxygen. Simply put, even ...
References
{{Weathering Polymers Materials degradation Plastics and the environment Ultraviolet radiation