triphenylcarbenium on:
[Wikipedia]
[Google]
[Amazon]
 In
In
Image:Methyl Violet 10B.png,
N. C. Deno, J. J. Jaruzelski, and Alan Schriesheim (1955) "Carbonium ions. I. An acidity function (''C''0) derived from arylcarbonium ion equilibria." ''Journal of the American Chemical Society'', voume 77, issue 11, pages 3044–3051.
Michael E. Jung, Roman Lagoutte, and Ullrich Jahn (2011): "Triphenylcarbenium Tetrafluoroborate". In ''Encyclopedia of Reagents for Organic Synthesis''.
E. Molins, M. Mas, W. Maniukiewicz, M. Ballester and J. Castañer (1996): "Perchlorotriphenylcarbenium Hexachloroantimonate(V)". ''Acta Crystallographica Section C (Structural Chemistry)'', volume C52, pages 2412-2414. {{doi, 10.1107/S0108270196007287
U. S. National Institutes of Health (2019)
PubChem ID 2723954 - Triphenylcarbenium hexafluorophosphate
. Entry in NCBI's PubChem database, accessed on 2019-07-25.
Carbocations

 In
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, proper ...
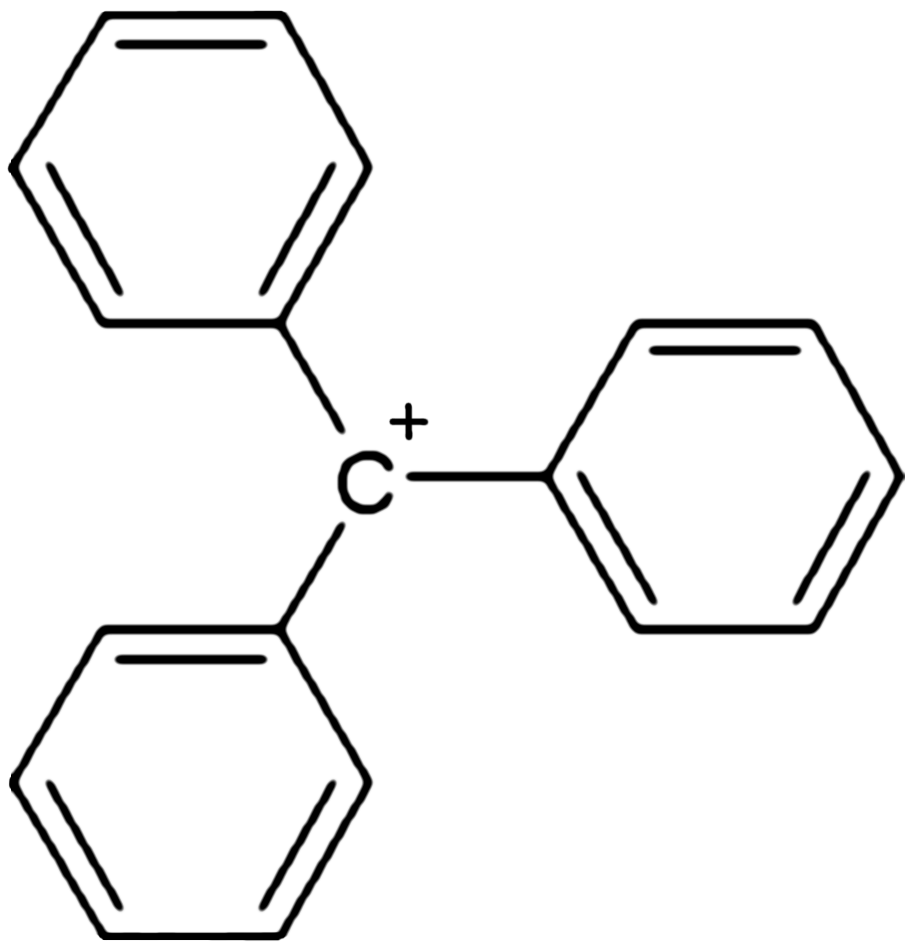
, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion with formula or , consisting of a carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
atom with a positive charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons resp ...
connected to three phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
groups. It is a charged version of the triphenylmethyl radical
The triphenylmethyl radical (often shorted to trityl radical) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trit ...
•. The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride
Triphenylmethyl chloride or trityl chloride (TrCl) is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.
Preparation
Triphenylmethyl chloride is commercially available. ...
that do not contain the cation.
Triphenylcarbenium is a relatively stable carbenium
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with a trivalent carbon that bears a +1 formal charge.
In older literature the name carbonium ion was used for this class, but now it refers exclusivel ...
ion, because the positive charge is partially distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom).
Derivatives
The cation exists in important chemicalreagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s and catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s such as triphenylmethyl hexafluorophosphate . Related salts are known with diverse anions including (), hexachloroantimonate (), and perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, . The majority of perchlorates are commercially produced salts. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. ...
(). This and other similar cations can be obtained as intensely colored solutions by dissolving aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
-substituted methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is ...
s in concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
. Derivatives of this cation include, for example, perchlorotriphenylcarbenium .
Triarylmethane dyes
Triarylmethane dyes are derivatives are stabilized version of the trityl cation. They are water-soluble and are often obtained as the chloride salts. These dyes have strong electron donor groups, often amines, at the ''p''-positions of two or three of the aryl groups.Thomas Gessner and Udo Mayer "Triarylmethane and Diarylmethane Dyes" in Ullmann's Encyclopedia of Industrial Chemistry 2002,Wiley-VCH
Wiley-VCH is a German publisher owned by John Wiley & Sons. It was founded in 1921 as Verlag Chemie (meaning "Chemistry Press": VCH stands for ''Verlag Chemie'') by two German learned societies. Later, it was merged into the German Chemical Soci ...
, Weinheim.
Crystal violet
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antif ...
.
Image:NewFuchsineStructure.png, New fuchsine dye.
File:Pararosaniline.png, Pararosaniline
See also
*Triphenylmethane
Triphenylmethane, or triphenyl methane, is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many synthetic dyes called triarylmeth ...
*Triphenylmethanol
Triphenylmethanol (also known as triphenylcarbinol, TrOH) is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. In strongly acidic solutions ...
References
PubChem ID 2723954 - Triphenylcarbenium hexafluorophosphate
. Entry in NCBI's PubChem database, accessed on 2019-07-25.