Transalkylation on:
[Wikipedia]
[Google]
[Amazon]
In
 Transalkylation, as used by the petrochemical industry, is often used to convert toluene into benzene and xylenes. This is achieved through a
Transalkylation, as used by the petrochemical industry, is often used to convert toluene into benzene and xylenes. This is achieved through a
C6H4(C2H5)2 + C6H6 -> 2 C6H5C2H5
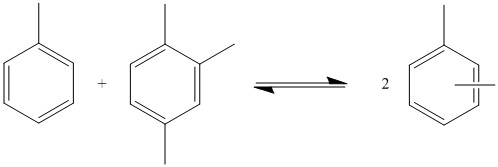 This type of reaction can also be performed with toluene and trimethylbenzene to produce xylene. The reaction occurs via equilibrium, so the product is not pure xylene. Many products are produced with varying numbers of methyl groups. The quantities in which each product is produced depends on the M/R ratio. This is the ratio of the number of methyl groups to the number of benzene rings in all of the substrates. For example, in the disproportionation of toluene, the M/R ratio is 1. Side reactions in which alkanes are produced reduce the number of methyl groups available which decreases the M/R ratio. This can be mitigated by adding compounds with higher numbers of methyl groups, such as trimethylbenzene. The ratio of products produced depends only on the M/R ratio so different starting materials can produce the same compounds via transalkylation.Tsai, Tseng-Chang "Disproportionation and Transalkylation of Alkylbenzenes over Zeolite Catalysts". Elsevier Science, 1999
This type of reaction can also be performed with toluene and trimethylbenzene to produce xylene. The reaction occurs via equilibrium, so the product is not pure xylene. Many products are produced with varying numbers of methyl groups. The quantities in which each product is produced depends on the M/R ratio. This is the ratio of the number of methyl groups to the number of benzene rings in all of the substrates. For example, in the disproportionation of toluene, the M/R ratio is 1. Side reactions in which alkanes are produced reduce the number of methyl groups available which decreases the M/R ratio. This can be mitigated by adding compounds with higher numbers of methyl groups, such as trimethylbenzene. The ratio of products produced depends only on the M/R ratio so different starting materials can produce the same compounds via transalkylation.Tsai, Tseng-Chang "Disproportionation and Transalkylation of Alkylbenzenes over Zeolite Catalysts". Elsevier Science, 1999
(CH3)3COC6H5 -> HOC6H4C(CH3)3
Additionally, 2,4-di-''tert''-butylphenol is converted to 4-''tert''-butylphenol by treatment with HOC6H3(C(CH3)3)2 + HOC6H5 -> 2 HOC6H4C(CH3)3
Transalkylation in conjunction with the 
Process and apparatus for ethylbenzene production and transalkylation to xyleneTransalkylation processExxon Mobil Transalkylation ProcessTransalkylation in the Petrochemical Industry
{{Functional Groups Addition reactions
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J ...
, transalkylation is a chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
involving the transfer of an alkyl group
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
from one organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
to another. The reaction is used for the transfer of methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in ...
and ethyl group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry's nomenclature of organic chemistry for a saturated ...
s between benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
rings. This is of particular value in the petrochemical industry
The petrochemical industry is concerned with the production and trade of petrochemicals. A major part is constituted by the plastics (polymer) industry. It directly interfaces with the petroleum industry, especially the downstream sector.
Comp ...
to manufacture p-xylene
''p''-Xylene ( ''para''-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The ''p-'' stands for ''para-'', indicating that the two methyl groups in ''p''-xylene occupy the diamet ...
, styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
, and other aromatic compounds. Motivation for using transalkylation reactions is based on a difference in production and demand for benzene, toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
, and xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are s ...
s. Transalkylation can convert toluene, which is overproduced, into benzene and xylene, which are under-produced. Zeolite
Zeolites are microporous, crystalline aluminosilicate materials commonly used as commercial adsorbents and catalysts. They mainly consist of silicon, aluminium, oxygen, and have the general formula ・y where is either a metal ion or H+. These p ...
s are often used as catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s in transalkylation reactions.
Disproportionation
 Transalkylation, as used by the petrochemical industry, is often used to convert toluene into benzene and xylenes. This is achieved through a
Transalkylation, as used by the petrochemical industry, is often used to convert toluene into benzene and xylenes. This is achieved through a disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can ...
reaction of toluene in which one toluene molecule transfers its methyl group to another one. The reaction is not selective, and the xylene produced can be ortho, meta, or para. There is a higher demand for para xylene, so it is often separated, and the mixture is allowed to reequilibrate to give more para product.
Diethylbenzenes
Diethylbenzenes arise as side-products of the alkylation of benzene with ethylene, which is conducted on a very large scale. Since there is only a limited market for diethylbenzene, much of it is recycled by transalkylation to give ethylbenzene: :M/R Ratio
 This type of reaction can also be performed with toluene and trimethylbenzene to produce xylene. The reaction occurs via equilibrium, so the product is not pure xylene. Many products are produced with varying numbers of methyl groups. The quantities in which each product is produced depends on the M/R ratio. This is the ratio of the number of methyl groups to the number of benzene rings in all of the substrates. For example, in the disproportionation of toluene, the M/R ratio is 1. Side reactions in which alkanes are produced reduce the number of methyl groups available which decreases the M/R ratio. This can be mitigated by adding compounds with higher numbers of methyl groups, such as trimethylbenzene. The ratio of products produced depends only on the M/R ratio so different starting materials can produce the same compounds via transalkylation.Tsai, Tseng-Chang "Disproportionation and Transalkylation of Alkylbenzenes over Zeolite Catalysts". Elsevier Science, 1999
This type of reaction can also be performed with toluene and trimethylbenzene to produce xylene. The reaction occurs via equilibrium, so the product is not pure xylene. Many products are produced with varying numbers of methyl groups. The quantities in which each product is produced depends on the M/R ratio. This is the ratio of the number of methyl groups to the number of benzene rings in all of the substrates. For example, in the disproportionation of toluene, the M/R ratio is 1. Side reactions in which alkanes are produced reduce the number of methyl groups available which decreases the M/R ratio. This can be mitigated by adding compounds with higher numbers of methyl groups, such as trimethylbenzene. The ratio of products produced depends only on the M/R ratio so different starting materials can produce the same compounds via transalkylation.Tsai, Tseng-Chang "Disproportionation and Transalkylation of Alkylbenzenes over Zeolite Catalysts". Elsevier Science, 1999
Zeolite catalysts
Transalkylation reactions of six to ten carbon methylated aromatics are often performed with the cofed of hydrogen gas, over a zeolite based solid catalyst. Industrial processes operate the transalkylation reactor at elevated temperature and pressure to achieve desired process economics. Zeolites are micro-crystalline solids composed of tetrahedral and building blocks. These crystals are porous in nature with characteristic micropore channels, cavities. Zeolite is known as one class of molecular sieve because of their channel openings are often between 0.4-1.5 nanometers, just enough for the molecules to pass through. Aromatics molecules enter and exit these channels at different rates, also called diffusion. In addition to their molecular sieving effect, zeolites have weakly bonded protons originated from its chemical composition. These are chemical active centers for acid-catalyzed transalkylation reaction. Zeolites of varying sizes are used to perform transalkylation on different substrates. For example, zeolites with a pore size of 5.5Å are suitable for benzene, toluene, xylenes and trimethylbenzenes transalkylations.Phenols
Transalkylation is employed in the commercial production of aromatics beyond the usual BTX feedstocks. For example, 4-''tert''-butylphenol is produced in part via two transalkylation reactions. In one example, ''tert''-butylphenyl ether is isomerized to the phenol: :phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it r ...
by transalkylation:
:Hock rearrangement
Hock may refer to:
Common meanings:
* Hock (wine), a type of wine
* Hock (anatomy), part of an animal's leg
* To leave an item with a pawnbroker
People:
* Hock (surname)
* Richard "Hock" Walsh (1948-1999), Canadian blues singer
Other uses:
* A ...
contributes to the production of 1,3-diisopropylbenzene, a precursor to resorcinol
Resorcinol (or resorcin) is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or '' meta''-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in ...
.
See also
*Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effectin ...
*BTX (chemistry)
In the petroleum refining and petrochemical industries, the initialism BTX refers to mixtures of benzene, toluene, and the three xylene isomers, all of which are aromatic hydrocarbons. The xylene isomers are distinguished by the designations ''o ...
*Friedel–Crafts reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation react ...
*Hydrodealkylation
Hydrodealkylation is a chemical reaction that often involves reacting an aromatic hydrocarbon, such as toluene, in the presence of hydrogen gas to form a simpler aromatic hydrocarbon devoid of functional groups. An example is the conversion of 1,2, ...
*Transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction ca ...
References
External links
Process and apparatus for ethylbenzene production and transalkylation to xylene
{{Functional Groups Addition reactions