Thermodynamic versus kinetic reaction control on:
[Wikipedia]
[Google]
[Amazon]
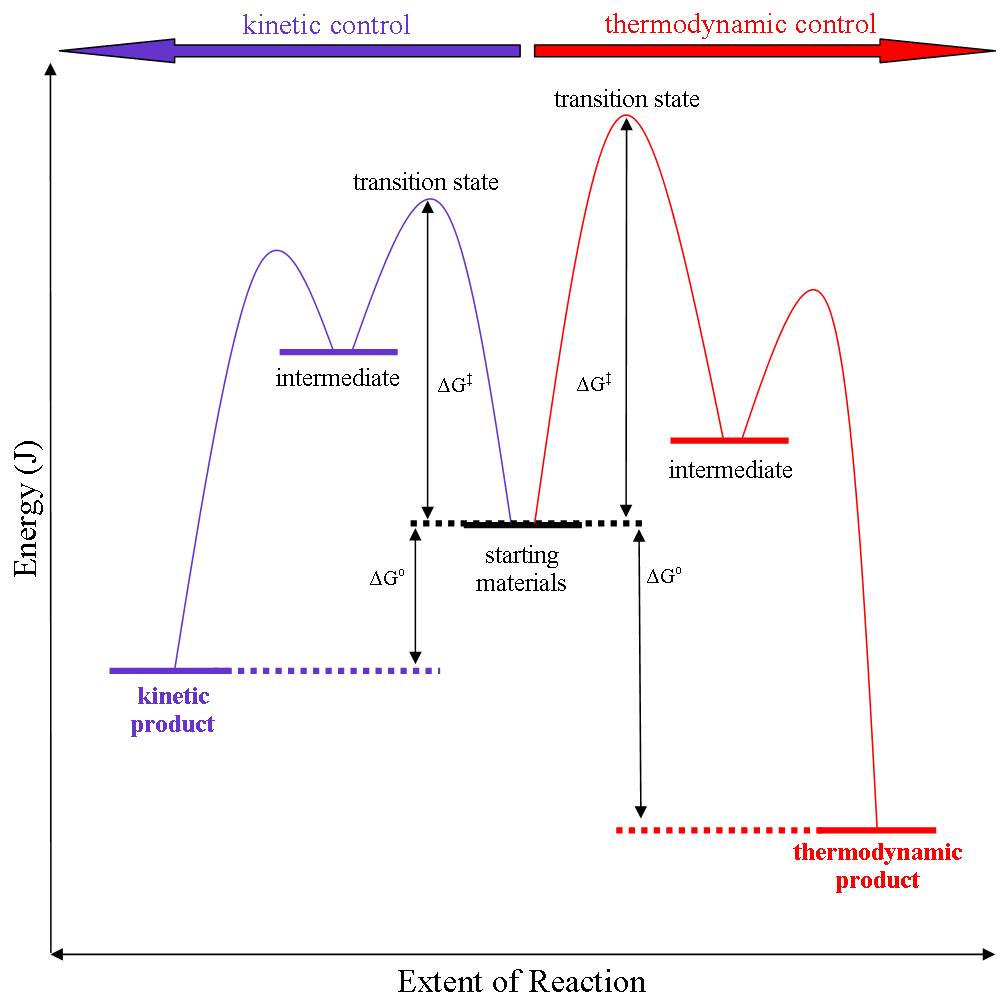 Thermodynamic reaction control or kinetic reaction control in a
Thermodynamic reaction control or kinetic reaction control in a
 An outstanding and very rare example of the ''full'' kinetic and thermodynamic reaction control in the process of the
An outstanding and very rare example of the ''full'' kinetic and thermodynamic reaction control in the process of the  Theoretical DFT calculations of the reaction between
Theoretical DFT calculations of the reaction between  Further, the reaction could proceed ''via'' two competing channels, ''i.e.'' either leading to the pincer type products 5 ''via'' TS2k or resulting in the formation of the domino product 6 ''via'' TS2t. The calculations showed that the first channel is more kinetically favourable (Δ''G''‡ ≈ 5.7–5.9 kcal/mol). Meanwhile, the domino products 6 are more thermodynamically stable than 5 (Δ''G''‡ ≈ 4.2-4.7 kcal/mol) and this fact may cause isomerization of 5 into 6 at elevated temperature. Indeed, the calculated activation barriers for the 5 → 6
Further, the reaction could proceed ''via'' two competing channels, ''i.e.'' either leading to the pincer type products 5 ''via'' TS2k or resulting in the formation of the domino product 6 ''via'' TS2t. The calculations showed that the first channel is more kinetically favourable (Δ''G''‡ ≈ 5.7–5.9 kcal/mol). Meanwhile, the domino products 6 are more thermodynamically stable than 5 (Δ''G''‡ ≈ 4.2-4.7 kcal/mol) and this fact may cause isomerization of 5 into 6 at elevated temperature. Indeed, the calculated activation barriers for the 5 → 6
/ref> Use of low temperatures and sterically demanding bases increases the kinetic selectivity. Here, the difference in p''K''b between the base and the enolate is so large that the reaction is essentially irreversible, so the equilibration leading to the thermodynamic product is likely a proton exchange occurring during the addition between the kinetic enolate and as-yet-unreacted ketone. An inverse addition (adding ketone to the base) with rapid mixing would minimize this. The position of the equilibrium will depend on the countercation and solvent. If a much weaker base is used, the deprotonation will be incomplete, and there will be an equilibrium between reactants and products. Thermodynamic control is obtained, however the reaction remains incomplete unless the product enolate is trapped, as in the example below. Since H transfers are very fast, the trapping reaction being slower, the ratio of trapped products largely mirrors the deprotonation equilibrium.
If a much weaker base is used, the deprotonation will be incomplete, and there will be an equilibrium between reactants and products. Thermodynamic control is obtained, however the reaction remains incomplete unless the product enolate is trapped, as in the example below. Since H transfers are very fast, the trapping reaction being slower, the ratio of trapped products largely mirrors the deprotonation equilibrium.

 :The rationale for the differing selectivities is as follows: Both products result from
:The rationale for the differing selectivities is as follows: Both products result from 
 C. K. Ingold with E. D. Hughes and G. Catchpole independently described a thermodynamic and kinetic reaction control model in 1948. They were reinvestigating a certain
C. K. Ingold with E. D. Hughes and G. Catchpole independently described a thermodynamic and kinetic reaction control model in 1948. They were reinvestigating a certain 
 Thermodynamic reaction control or kinetic reaction control in a
Thermodynamic reaction control or kinetic reaction control in a chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breakin ...
can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity
Selectivity may refer to:
Psychology and behaviour
* Choice, making a selection among options
* Discrimination, the ability to recognize differences
* Socioemotional selectivity theory, in social psychology
Engineering
* Selectivity (radio), a ...
or stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation o ...
. The distinction is relevant when product A forms faster than product B because the activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000
The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ''E''a ( energy of activation) than the other.
Prevalence of thermodynamic or kinetic control determines the final composition of the product when these competing reaction pathways lead to different products. The reaction conditions as mentioned above influence the selectivity
Selectivity may refer to:
Psychology and behaviour
* Choice, making a selection among options
* Discrimination, the ability to recognize differences
* Socioemotional selectivity theory, in social psychology
Engineering
* Selectivity (radio), a ...
of the reaction - i.e., which pathway is taken.
Asymmetric synthesis is a field in which the distinction between kinetic and thermodynamic control is especially important. Because pairs of enantiomers have, for all intents and purposes, the same Gibbs free energy, thermodynamic control will produce a racemic mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
by necessity. Thus, any ''catalytic'' reaction that provides product with nonzero enantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a sin ...
is under at least partial kinetic control. (In many ''stoichiometric'' asymmetric transformations, the enantiomeric products are actually formed as a complex with the chirality source before the workup stage of the reaction, technically making the reaction a diastereoselective one. Although such reactions are still usually kinetically controlled, thermodynamic control is at least possible, in principle.)
Scope
In Diels–Alder reactions
TheDiels–Alder reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexe ...
of cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often abbreviated CpH beca ...
with furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highl ...
can produce two isomeric
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
products. At room temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on ...
, kinetic reaction control prevails and the less stable endo isomer
In organic chemistry, ''endo''–''exo'' isomerism is a special type of stereoisomerism found in organic compounds with a substituent on a bridged ring system. The prefix ''endo'' is reserved for the isomer with the substituent located closest, ...
2 is the main reaction product. At 81 °C and after long reaction times, the chemical equilibrium can assert itself and the thermodynamically more stable exo isomer 1 is formed. The ''exo'' product is more stable by virtue of a lower degree of steric congestion, while the ''endo'' product is favoured by orbital overlap in the transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
.
tandem
Tandem, or in tandem, is an arrangement in which a team of machines, animals or people are lined up one behind another, all facing in the same direction.
The original use of the term in English was in ''tandem harness'', which is used for two ...
inter-/intramolecular Diels–Alder reaction of bis-furyl dienes 3 with hexafluoro-2-butyne
Hexafluoro-2-butyne (HFB) is a fluorocarbon with the chemical structure CF3C≡CCF3. HFB is a particularly electrophilic acetylene derivative, and hence a potent dienophile for Diels–Alder reactions.
HFB is prepared by the action of sulfur te ...
or dimethyl acetylenedicarboxylate (DMAD) have been discovered and described in 2018. At low temperature, the reactions occur chemoselectively leading exclusively to adducts of pincer- +2cycloaddition (5). The exclusive formation of domino
Dominoes is a family of tile-based games played with gaming pieces, commonly known as dominoes. Each domino is a rectangular tile, usually with a line dividing its face into two square ''ends''. Each end is marked with a number of spots (also ca ...
-adducts (6) is observed at elevated temperatures.
hexafluoro-2-butyne
Hexafluoro-2-butyne (HFB) is a fluorocarbon with the chemical structure CF3C≡CCF3. HFB is a particularly electrophilic acetylene derivative, and hence a potent dienophile for Diels–Alder reactions.
HFB is prepared by the action of sulfur te ...
and dienes 3a-c were performed. The reaction starting with +2cycloaddition of CF3C≡CCF3 at one of the furan moieties occurs in a concerted fashion ''via'' TS1 and represents the rate limiting step of the whole process with the activation barrier Δ''G''‡ ≈ 23.1–26.8 kcal/mol.
isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeri ...
''via'' the retro-Diels–Alder reaction of 5 followed by the intramolecular +2cycloaddition in the chain intermediate 4 to give 6 are 34.0–34.4 kcal/mol.
In enolate chemistry
In theprotonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, i ...
of an enolate ion, the kinetic product is the enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The ter ...
and the thermodynamic product is a ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
or aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
. Carbonyl compounds
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
and their enols interchange rapidly by proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
transfers catalyzed by acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
s or bases, even in trace amounts, in this case mediated by the enolate or the proton source.
In the deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.edu ...
of an unsymmetrical ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
, the kinetic product is the enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
resulting from removal of the most accessible α-H while the thermodynamic product has the more highly substituted enolate moiety.Thermodynamic Product vs Kinetic Product/ref> Use of low temperatures and sterically demanding bases increases the kinetic selectivity. Here, the difference in p''K''b between the base and the enolate is so large that the reaction is essentially irreversible, so the equilibration leading to the thermodynamic product is likely a proton exchange occurring during the addition between the kinetic enolate and as-yet-unreacted ketone. An inverse addition (adding ketone to the base) with rapid mixing would minimize this. The position of the equilibrium will depend on the countercation and solvent.
In electrophilic additions
Theelectrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic Che ...
reaction of hydrogen bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room tempe ...
to 1,3-butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two viny ...
above room temperature leads predominantly to the thermodynamically more stable 1,4 adduct, 1-bromo-2-butene, but decreasing the reaction temperature to below room temperature favours the kinetic 1,2 adduct, 3-bromo-1-butene.
Markovnikov Markovnikov (russian: Марковников) is a Russian masculine surname, which originates from ''морковь'' (''carrot''); its feminine counterpart is Markovnikova. It may refer to
*Vladimir Markovnikov (1837–1904), Russian chemist
* Nik ...
protonation at position 1, resulting in a resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillati ...
-stabilized allylic cation. The 1,4 adduct places the larger Br atom at a less congested site and includes a more highly substituted alkene moiety, while the 1,2 adduct is the result of the attack by the nucleophile (Br−) at the carbon of the allylic cation bearing the greatest positive charge (the more highly substituted carbon is the most likely place for the positive charge).
Characteristics
* In principle, every reaction is on the continuum between pure kinetic control and pure thermodynamic control. These terms are with respect to a given temperature and time scale. A process approaches pure kinetic control at low temperature and short reaction time. For a sufficiently long time scale, every reaction approaches pure thermodynamic control, at least in principle. This time scale becomes shorter as the temperature is raised. *In every reaction, the first product formed is that which is most easily formed. Thus, every reaction ''a priori'' starts under kinetic control. * A necessary condition for thermodynamic control is reversibility or a mechanism permitting the equilibration between products. Reactions are considered to take place under thermodynamic reaction control when the reverse reaction is sufficiently rapid that the equilibrium establishes itself within the allotted reaction time. In this way, the thermodynamically more stable product is always favoured. * Under kinetic reaction control, one or both forward reactions leading to the possible products is significantly faster than the equilibration between the products. After reaction time ''t'', the product ratio is the ratio of rate constants ''k'' and thus a function of the difference in activation energies ''E''a or Δ''G''‡: : (equation 1) :Unless equilibration is prevented (e.g., by removal of the product from the reaction mixture as soon as it forms), "pure" kinetic control is strictly speaking impossible, because some amount of equilibration will take place before the reactants are entirely consumed. In practice, many systems are well approximated as operating under kinetic control, due to negligibly slow equilibration. For example, many enantioselective catalytic systems provide nearly enantiopure product (> 99% ee), even though the enantiomeric products have the same Gibbs free energy and are equally favored thermodynamically. * Under pure thermodynamic reaction control, when the equilibrium has been reached, the product distribution will be a function of the stabilities ''G''°. After an infinite amount of reaction time, the ratio of product concentrations will equal theequilibrium constant
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency ...
''K''eq and therefore be a function of the difference in Gibbs free energies,
: (equation 2)
:In principle, "pure" thermodynamic control is also impossible, since equilibrium is only achieved after infinite reaction time. In practice, if A and B interconvert with overall rate constants ''k''f and ''k''r, then for most practical purposes, the change in composition becomes negligible after ''t'' ~ 3.5/(''k''f + ''k''r), or approximately five half-lives, and the system product ratio can be regarded as the result of thermodynamic control.
* In general, short reaction times favour kinetic control, whereas longer reaction times favour thermodynamic reaction control. Low temperatures will enhance the selectivity under both sets of conditions, since ''T'' is in the denominator in both cases. The ideal temperature to optimise the yield of the fastest-forming product will be the lowest temperature that will ensure reaction completion in a reasonable amount of time. The ideal temperature for a reaction under thermodynamic control is the lowest temperature at which equilibrium will be reached in a reasonable amount of time. If needed, the selectivity can be increased by then slowly cooling the reaction mixture to shift the equilibrium further toward the most stable product. When the difference in product stability is very large, the thermodynamically controlled product can dominate even under relatively vigorous reaction conditions.
* If a reaction is under thermodynamic control at a given temperature, it will also be under thermodynamic control at a higher temperature for the same reaction time.
* In the same manner, if a reaction is under kinetic control at a given temperature, it will also be under kinetic control at any lower temperature for the same reaction time.
* If one presumes that a new reaction will be ''a priori'' under kinetic control, one can detect the presence of an equilibration mechanism (and therefore the possibility of thermodynamic control) if the product distribution:
** changes over time,
** shows one product to be dominant at one temperature while another dominates at a different temperature (inversion of dominance), or
** changes with temperature but is not consistent with equation 1, that is a change in temperature (without changing the reaction time) causes a change in the product ratio that is larger or smaller than would be expected from the change in temperature alone, assuming that is largely invariant with temperature over a modest temperature range.
* In the same way, one can detect the possibility of kinetic control if a temperature change causes a change in the product ratio that is inconsistent with equation 2, assuming that is largely invariant with temperature over a modest temperature range.
History
The first to report on the relationship between kinetic and thermodynamic control were R.B. Woodward and Harold Baer in 1944. They were re-investigating a reaction betweenmaleic anhydride
Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and pol ...
and a fulvene
Fulvene (pentafulvene) is a hydrocarbon with the formula (CH=CH)2C=CH2. It is a prototype of a cross-conjugated hydrocarbon. Fulvene is rarely encountered, but substituted derivatives ( fulvenes) are numerous. They are mainly of interest as ligand ...
first reported in 1929 by Otto Diels
Otto Paul Hermann Diels (; 23 January 1876 – 7 March 1954) was a German chemist. His most notable work was done with Kurt Alder on the Diels–Alder reaction, a method for diene synthesis. The pair was awarded the Nobel Prize in Chemi ...
and Kurt Alder
Kurt Alder (; 10 July 1902 – 20 June 1958) was a German chemist and Nobel laureate.
Biography
Alder was born in the industrial area of Königshütte, Silesia (modern day Chorzów, Upper Silesia, Poland), where he received his early scho ...
. They observed that ''while the endo isomer is formed more rapidly, longer reaction times, as well as relatively elevated temperatures, result in higher exo / endo ratios'' which had to be '' considered in the light of the remarkable stability of the exo-compound on the one hand and the very facile dissociation of the endo isomer on the other.''
:allylic rearrangement An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution.
In reaction conditions that favor a SN1 reactio ...
reported in 1930 by Jakob Meisenheimer.Meisenheimer, J. and Link, J. (1930), ''Über die Verschiebung in der Allyl-Gruppe. 3. Mitteilung über Substitution und Addition''. Justus Liebigs Annalen der Chemie, 479: 211–277. Solvolysis of gamma-phenylallyl chloride with AcOK in acetic acid was found to give a mixture of the gamma and the alpha acetate with the latter converting to the first by equilibration. This was interpreted as a ''case in the field of anionotropy of the phenomenon, familiar in prototropy, of the distinction between kinetic and thermodynamic control in ion-recombination''.
:References
{{reflist Chemical reactions Thermodynamics Chemical thermodynamics