Triisopropylamine on:
[Wikipedia]
[Google]
[Amazon]
Triisopropylamine is an organic
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
consisting of three isopropyl
In organic chemistry, a propyl group is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent ...
groups bound to a central nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
. As a hindered tertiary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
, it can be used as a non-nucleophilic base and as a stabilizer for polymers; however, its applications are limited by its relatively high cost and difficult synthesis.
Structure
In the early 1990s, theoretical studies andelectron diffraction
Electron diffraction is a generic term for phenomena associated with changes in the direction of electron beams due to elastic interactions with atoms. It occurs due to elastic scattering, when there is no change in the energy of the electrons. ...
analysis of the 3D structure of the molecule, in the gas phase or in non-polar solvents, indicated that the bonds between the nitrogen atom and the three carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms were nearly coplanar in the ground state, instead of forming a trigonal pyramid
In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry). When all three atoms at the corner ...
as in simpler amines. The average C-N-C angle was claimed to be 119.2°, much closer to the 120° of the flat configuration than to the 111.8° of trimethylamine
Trimethylamine (TMA) is an organic compound with the formula N(CH3)3. It is a trimethylated derivative of ammonia. TMA is widely used in industry. At higher concentrations it has an ammonia-like odor, and can cause necrosis of mucous membranes ...
. This peculiarity was attributed to steric hindrance
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
by the bulky isopropyl radicals. However, in 1998 X-ray diffraction
X-ray diffraction is a generic term for phenomena associated with changes in the direction of X-ray beams due to interactions with the electrons around atoms. It occurs due to elastic scattering, when there is no change in the energy of the waves. ...
analysis of the crystallized solid showed that the C3N core is actually pyramidal, with the N atom lying approximately 0.28 Å off the carbons' plane (whereas in trimethylamine the distance is about 0.45 Å). However the researchers could not rule out the crystal field effect as the cause of the asymmetry.
The C-C-C planes of the isopropyl groups are slightly tilted (about 5°) relative to the threefold symmetry axis of the C3N core.
Even more hindered amines
Triisopropylamine is notable as being among the most sterically hindered amines currently known. To date, di(''tert''-alkyl)(''sec''-alkyl)amines like di-''tert''-butyl(isopropyl)amine (''t''Bu2iPrN) and diadamantylcyclohexylamine (Ad2CyN) have been prepared in low yield, as have a handful of tri-''tert''-alkylamines in which two of the ''tert''-alkyl groups are tied together in a five-membered ring. As a result of the extreme steric crowding, these tertiary amines were found to be unusually susceptible to β-elimination to give an alkene and a secondary amine, even at temperatures close to room temperature (as low as 40 °C). As a result of these and other synthetic difficulties, the authors of a 2018 study predict that the even more crowded tri''-tert-''butylamine (''t''Bu3N, unknown as of 2023) will likely remain a longstanding unsolved synthetic challenge, even though ''ab initio'' calculations indicate that it would be of reasonable stability if it could be prepared. Calculations indicate that tri-''tert''-butylamine should be planar at nitrogen. Though arguably a more hindered molecule, the structurally analogous tri-''tert''-alkyl carbinol 2,2,4,4-tetramethyl-3-''t''-butyl-pentane-3-ol (tri-''tert''-butylcarbinol, ''t''Bu3COH), has been known for decades (prepared by Bartlett and coworkers in 1945).Preparation
Steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
make triisopropylamine difficult to synthesise and unlike less hindered tertiary amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s (such as triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
) it cannot be produced by the alkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
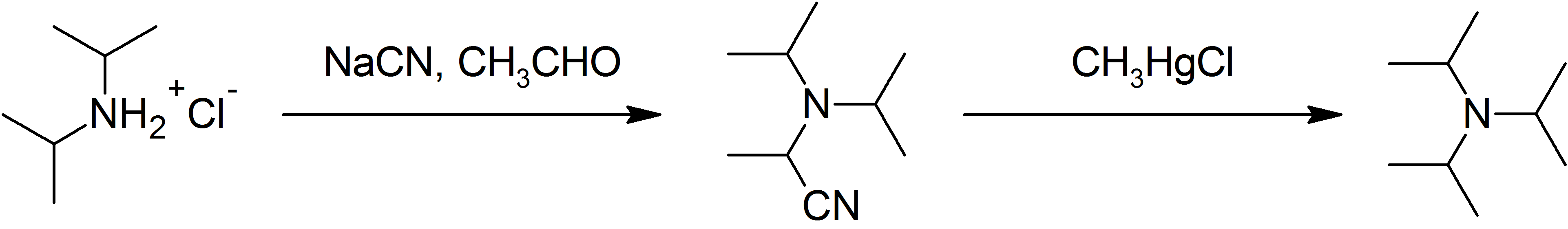
of ammonia with alcohol; attempts to do so stall at diisopropylamine. It can be prepared from diisopropylamine on the laboratory scale:
:
See also
* Tetra-''tert''-butylethyleneReferences
{{reflist Non-nucleophilic bases Diisopropylamino compounds Tertiary amines