Thoriated Filament on:
[Wikipedia]
[Google]
[Amazon]
 In
In



/ref>
John Harper (2003) ''Tubes 201 - How vacuum tubes really work'', John Harper's home page
* {{Thermionic valves Electrodes Gas discharge lamps Vacuum tubes Accelerator physics
 In
In vacuum tube
A vacuum tube, electron tube, thermionic valve (British usage), or tube (North America) is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. It ...
s and gas-filled tube
A gas-filled tube, also commonly known as a discharge tube or formerly as a Julius Plücker, Plücker tube, is an arrangement of electrodes in a gas within an dielectric, insulating, temperature-resistant envelope. Gas-filled tubes exploit phen ...
s, a hot cathode or thermionic cathode is a cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
electrode which is heated to make it emit electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s due to thermionic emission
Thermionic emission is the liberation of charged particles from a hot electrode whose thermal energy gives some particles enough kinetic energy to escape the material's surface. The particles, sometimes called ''thermions'' in early literature, a ...
. This is in contrast to a cold cathode
A cold cathode is a cathode that is not electrically heated by a Electrical filament, filament.A negatively charged electrode emits electrons or is the positively charged terminal. For more, see field emission. A cathode may be considered "cold" ...
, which does not have a heating element. The heating element is usually an electrical filament
An incandescent light bulb, also known as an incandescent lamp or incandescent light globe, is an electric light that produces illumination by Joule heating a filament until it glows. The filament is enclosed in a glass bulb that is eith ...
heated by a separate electric current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge c ...
passing through it. Hot cathodes typically achieve much higher power density than cold cathodes, emitting significantly more electrons from the same surface area. Cold cathode
A cold cathode is a cathode that is not electrically heated by a Electrical filament, filament.A negatively charged electrode emits electrons or is the positively charged terminal. For more, see field emission. A cathode may be considered "cold" ...
s rely on field electron emission
Field electron emission, also known as field-induced electron emission, field emission (FE) and electron field emission, is the emission of electrons from a material placed in an electrostatic field. The most common context is field emission from ...
or secondary electron
Secondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated by other radiation (the ''primary'' radiation). This radiation can be in the form of ions, electrons, or photons with suffi ...
emission from positive ion bombardment, and do not require heating. There are two types of hot cathode. In a ''directly heated cathode'', the filament is the cathode and emits the electrons. In an ''indirectly heated cathode'', the filament or ''heater'' heats a separate metal cathode electrode which emits the electrons.
From the 1920s to the 1960s, a wide variety of electronic devices used hot-cathode vacuum tubes. Today, hot cathodes are used as the source of electrons in fluorescent lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, to produce ultraviolet and make a phosphor ...
s, vacuum tube
A vacuum tube, electron tube, thermionic valve (British usage), or tube (North America) is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. It ...
s, and the electron gun
file:Egun.jpg, Electron gun from a cathode-ray tube
file:Vidicon Electron Gun.jpg, The electron gun from an RCA Vidicon video camera tube
An electron gun (also called electron emitter) is an electrical component in some vacuum tubes that produc ...
s used in cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a ...
s and laboratory equipment such as electron microscope
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing it ...
s.
Description
Acathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
electrode in a vacuum tube or other vacuum system is a metal surface which emits electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s into the evacuated space of the tube. Since the negatively charged electrons are attracted to the positive nuclei of the metal atoms, they normally stay inside the metal and require energy to leave it.
This energy is called the ''work function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
'' of the metal. In a hot cathode, the cathode surface is induced to emit electrons by heating it with a filament
The word filament, which is descended from Latin ''filum'' meaning " thread", is used in English for a variety of thread-like structures, including:
Astronomy
* Galaxy filament, the largest known cosmic structures in the universe
* Solar filament ...
, a thin wire of refractory metal
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definitions of which elements belong to this group di ...
like tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
with current flowing through it.Ferris, Clifford "Electron tube fundamentals" in
The cathode is heated to a temperature that causes electrons to be 'boiled off' of its surface into the evacuated space in the tube, a process called ''thermionic emission
Thermionic emission is the liberation of charged particles from a hot electrode whose thermal energy gives some particles enough kinetic energy to escape the material's surface. The particles, sometimes called ''thermions'' in early literature, a ...
''.
There are two types of hot cathodes:
;Directly heated cathode: In this type, the filament itself is the cathode, emits the electrons directly and is coated in metal oxides. Directly heated cathodes were used in the first vacuum tubes. Today, they are used in fluorescent tube
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, to produce ultraviolet and make a phosphor ...
s and most high-power transmitting vacuum tubes.
;Indirectly heated cathode: In this type, the filament is not the cathode but rather heats a separate cathode consisting of a sheet metal cylinder surrounding the filament, and the cylinder emits electrons. Indirectly heated cathodes are used in most low power vacuum tubes. For example, in most vacuum tubes the cathode is a nickel tube, coated with metal oxides. It is heated by a tungsten filament inside it, and the heat from the filament causes the outside surface of the oxide coating to emit electrons. The filament of an indirectly heated cathode is usually called the ''heater''.
The main reason for using an indirectly heated cathode is to isolate the rest of the vacuum tube from the electric potential across the filament, allowing vacuum tubes to use alternating current
Alternating current (AC) is an electric current that periodically reverses direction and changes its magnitude continuously with time, in contrast to direct current (DC), which flows only in one direction. Alternating current is the form in w ...
to heat the filament. In a tube in which the filament itself is the cathode, the alternating electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
from the filament surface would affect the movement of the electrons and introduce hum into the tube output. It also allows the filaments in all the tubes in an electronic device to be tied together and supplied from the same current source, even though the cathodes they heat may be at different potentials.
To improve electron emission, cathodes are usually treated with chemicals, compounds of metals with a low work function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" ...
. These form a metal layer on the surface which emits more electrons. Treated cathodes require less surface area, lower temperatures and less power to supply the same cathode current. The untreated thoriated tungsten filaments used in early vacuum tubes (called "bright emitters") had to be heated to , white-hot, to produce sufficient thermionic emission for use, while modern coated cathodes (called "dull emitters") produce far more electrons at a given temperature, so they only have to be heated to .
Types
Oxide-coated cathodes
The most common type of indirectly heated cathode is the oxide-coated cathode, in which the nickel cathode surface has a coating ofalkaline earth metal
The alkaline earth metals are six chemical elements in group (periodic table), group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar p ...
oxide to increase emission. One of the earliest materials used for this was barium oxide
Barium oxide, also known as baria, is a white hygroscopic non-flammable chemical compound, compound with the formula BaO. It has a Cubic crystal system, cubic structure and is used in cathode-ray tubes, crown glass, and Catalysis, catalysts. It ...
; it forms a monatomic layer of barium
Barium is a chemical element; it has symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
with an extremely low work function. More modern formulations utilize a mixture of barium oxide, strontium oxide
Strontium oxide or strontia, SrO, is formed when strontium reacts with oxygen. Burning strontium in air results in a mixture of strontium oxide and strontium nitride. It also forms from the decomposition of strontium carbonate SrCO3. It is a st ...
and calcium oxide
Calcium oxide (formula: Ca O), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term '' lime'' connotes calcium-containing ...
. Another standard formulation is barium oxide, calcium oxide, and aluminium oxide
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several Aluminium oxide (compounds), aluminium oxides, and specifically identified as alum ...
in a 5:3:2 ratio. Thorium oxide Thorium oxide may refer to:
* Thorium monoxide (thorium(II) oxide), ThO
* Thorium dioxide
Thorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in colour. Also known as thoria, it is mainly a by-pro ...
may be used as well. Oxide-coated cathodes operate at about 800-1000 °C, orange-hot. They are used in most small glass vacuum tubes, but are rarely used in high-power tubes because the coating is degraded by positive ions that bombard the cathode, accelerated by the high voltage on the tube.
For manufacturing convenience, the oxide-coated cathodes are usually coated with carbonate
A carbonate is a salt of carbonic acid, (), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word "carbonate" may also refer to a carbonate ester, an organic compound containing the carbonate group ...
s, which are then converted to oxides by heating. The activation may be achieved by microwave heating
Dielectric heating, also known as electronic heating, radio frequency heating, and high-frequency heating, is the process in which a radio frequency (RF) alternating electric field, or radio wave or microwave electromagnetic radiation heats a diel ...
, direct electric current heating, or electron bombardment while the tube is on the exhausting machine, until the production of gases ceases. The purity of cathode materials is crucial for tube lifetime. The Ba content significantly increases on the surface layers of oxide cathodes down to several tens of nanometers in depth, after the cathode activation process. The lifetime of oxide cathodes can be evaluated with a stretched exponential function
The stretched exponential function f_\beta (t) = e^ is obtained by inserting a fractional power law into the exponential function. In most applications, it is meaningful only for arguments between 0 and +∞. With , the usual exponential functi ...
. The survivability of electron emission sources is significantly improved by high doping of high‐speed activator.
Barium oxide reacts with traces of silicon in the underlying metal, forming a barium silicate (Ba2SiO4) layer. This layer has high electrical resistance, especially under discontinuous current load, and acts as a resistor in series with the cathode. This is particularly undesirable for tubes used in computer applications, where they can stay without conducting current for extended periods of time.Electron Tube Design, Radio Corporation of America, 1962
Barium also sublimates from the heated cathode, and deposits on nearby structures. For electron tubes, where the grid is subjected to high temperatures and barium contamination would facilitate electron emission from the grid itself, higher proportion of calcium is added to the coating mix (up to 20% of calcium carbonate).

Boride cathodes


Lanthanum hexaboride
Lanthanum hexaboride ( La B6, also called lanthanum boride and LaB) is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material that has a melting point of 2210 °C, and is insoluble in water and hydrochloric acid. ...
(LaB6) and cerium hexaboride
Cerium hexaboride ( Ce B6, also called cerium boride, CeBix, CEBIX, and (incorrectly) CeB) is an inorganic chemical, a boride of cerium. It is a refractory ceramic material. It has a low work function, one of the highest electron emissivities know ...
(CeB6) are used as the coating of some high-current cathodes. Hexaborides show low work function, around 2.5 eV. They are also resistant to poisoning. Cerium boride cathodes show lower evaporation rate at 1700 K than lanthanum boride, but it becomes equal at 1850 K and higher. Cerium boride cathodes have one and a half times the lifetime of lanthanum boride, due to its higher resistance to carbon contamination. Boride cathodes are about ten times as "bright" as the tungsten ones and have 10-15 times longer lifetime. They are used e.g. in electron microscope
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing it ...
s, microwave tube
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequencies between 300 MHz and ...
s, electron lithography
Electron-beam lithography (often abbreviated as e-beam lithography or EBL) is the practice of scanning a focused beam of electrons to draw custom shapes on a surface covered with an electron-sensitive film called a resist (exposing). The electron ...
, electron beam welding
Electron-beam welding (EBW) is a fusion welding process in which a charged-particle beam, beam of high-velocity electrons is applied to two materials to be joined. The workpieces melt and flow together as the kinetic energy of the electrons is ...
, X-Ray tube
An X-ray tube is a vacuum tube that converts electrical input power into X-rays. The availability of this controllable source of X-rays created the field of radiography, the imaging of partly opaque objects with penetrating radiation. In contras ...
s, and free electron laser
A free-electron laser (FEL) is a fourth generation light source producing extremely brilliant and short pulses of radiation. An FEL functions much as a laser but employs relativistic electrons as a gain medium instead of using stimulated emission ...
s. However these materials tend to be expensive.
Other hexaborides can be employed as well; examples are calcium hexaboride
Calcium hexaboride (sometimes calcium boride) is a compound of calcium and boron with the chemical formula CaB6. It is an important material due to its high electrical conductivity , hardness, chemical stability, and melting point. It is a black, l ...
, strontium hexaboride
Strontium boride ( Sr B6) is an inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of ...
, barium hexaboride, yttrium hexaboride, gadolinium hexaboride
Gadolinium is a chemical element; it has symbol Gd and atomic number 64. It is a silvery-white metal when oxidation is removed. Gadolinium is a malleable and ductile rare-earth element. It reacts with atmospheric oxygen or moisture slowly to form ...
, samarium hexaboride, and thorium hexaboride.
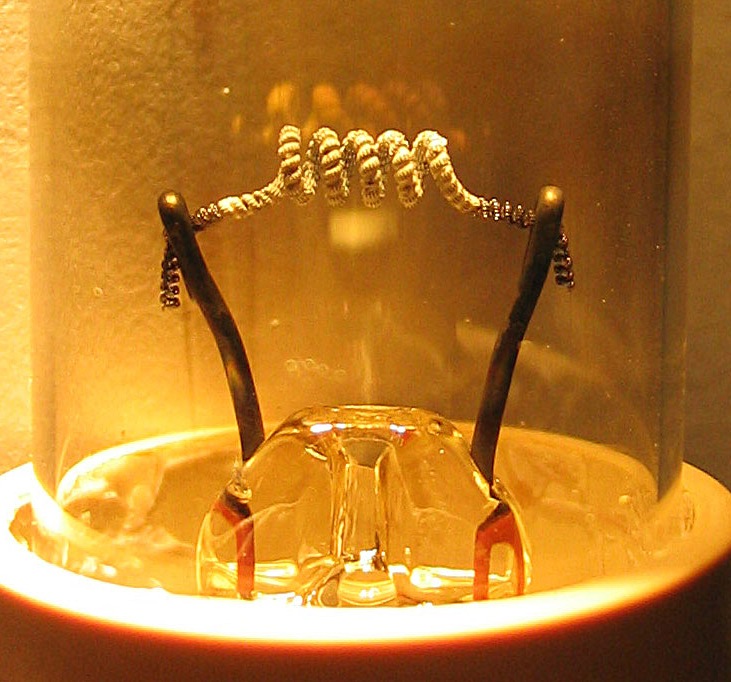
Thoriated filaments
A common type of directly heated cathode, used in most high power transmitting tubes, is the thoriated tungsten filament, discovered in 1914 and made practical byIrving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and metallurgical engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry.
Langmuir's most famous publicatio ...
in 1923. A small amount of thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
is added to the tungsten of the filament. The filament is heated white-hot, at about 2400 °C, and thorium atoms migrate to the surface of the filament and form the emissive layer. Heating the filament in a hydrocarbon atmosphere carburizes the surface and stabilizes the emissive layer. Thoriated filaments can have very long lifetimes and are resistant to the ion bombardment that occurs at high voltages, because fresh thorium continually diffuses to the surface, renewing the layer. They are used in nearly all high-power vacuum tubes for radio transmitters, and in some tubes for hi-fi
High fidelity (hi-fi or, rarely, HiFi) is the high-quality reproduction of sound. It is popular with audiophiles and home audio enthusiasts. Ideally, high-fidelity equipment has inaudible noise and distortion, and a flat (neutral, uncolored) ...
amplifiers. Their lifetimes tend to be longer than those of oxide cathodes.
Thorium alternatives
Due to concerns about thorium radioactivity and toxicity, efforts have been made to find alternatives. One of them is zirconiated tungsten, wherezirconium dioxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zirconium silicate or zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral bad ...
is used instead of thorium dioxide. Other replacement materials are lanthanum(III) oxide
Lanthanum(III) oxide, also known as lanthana, chemical formula , is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock ...
, yttrium(III) oxide
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance.
The thermal conductivity of yttrium oxide is 27 W/(m·K).
Applications Phosphors
Yttrium oxide is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors th ...
, cerium(IV) oxide
Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial produc ...
, and their mixtures.Electron emission materials and components: United States Patent 5911919/ref>
Other materials
In addition to the listed oxides and borides, other materials can be used as well. Some examples arecarbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
s and boride
A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some b ...
s of transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
s, e.g. zirconium carbide
Zirconium carbide () is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering.
Properties
It appears as a gray metallic powder with cubic crystal structure. It is ...
, hafnium carbide
Hafnium carbide () is a chemical compound of hafnium and carbon. Previously the material was estimated to have a melting point of about 3,900 °C. More recent tests have been able to conclusively prove that the substance has an even higher m ...
, tantalum carbide
Tantalum carbides (TaC) form a family of binary chemical compounds of tantalum and carbon with the empirical formula , where ''x'' usually varies between 0.4 and 1. They are extremely hard, brittle, refractory ceramic materials with metallic elec ...
, hafnium diboride
Hafnium diboride is a type of ceramic composed of hafnium and boron that belongs to the class of ultra-high temperature ceramics. It has a melting temperature of about 3250 °C. It is an unusual ceramic, having relatively high thermal and electr ...
, and their mixtures. Metals from groups
A group is a number of persons or things that are located, gathered, or classed together.
Groups of people
* Cultural group, a group whose members share the same cultural identity
* Ethnic group, a group whose members share the same ethnic iden ...
IIIB (scandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lantha ...
, yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost a ...
, and some lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
s, often gadolinium
Gadolinium is a chemical element; it has Symbol (chemistry), symbol Gd and atomic number 64. It is a silvery-white metal when oxidation is removed. Gadolinium is a malleable and ductile rare-earth element. It reacts with atmospheric oxygen or moi ...
and samarium
Samarium is a chemical element; it has symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of s ...
) and IVB (hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
, zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
, titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
) are usually chosen.
In addition to tungsten, other refractory metal
Refractory metals are a class of metals that are extraordinarily resistant to heat and wear. The expression is mostly used in the context of materials science, metallurgy and engineering. The definitions of which elements belong to this group di ...
s and alloys can be used, e.g. tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
, molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
and rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
and their alloys.
A barrier layer
A diffusion barrier is a thin layer (usually micrometres thick) of metal usually placed between two other metals. It is done to act as a barrier to protect either one of the metals from corrupting the other.
Adhesion of a plated metal layer to i ...
of other material can be placed between the base metal and the emission layer, to inhibit chemical reaction between these. The material has to be resistant to high temperatures, have high melting point and very low vapor pressure, and be electrically conductive. Materials used can be e.g. tantalum diboride, titanium diboride
Titanium diboride (TiB2) is an extremely hard ceramic which has excellent heat conductivity, oxidation stability and wear resistance. TiB2 is also a reasonable electrical conductor,J. Schmidt et al. "Preparation of titanium diboride TiB2 by spark p ...
, zirconium diboride
Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra-high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ...
, niobium diboride Niobium diboride (NbB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure.
Preparation
NbB2 can be synthesized by stoichiometric reaction between constituent elements, in this case Nb and B. This reaction pro ...
, tantalum carbide
Tantalum carbides (TaC) form a family of binary chemical compounds of tantalum and carbon with the empirical formula , where ''x'' usually varies between 0.4 and 1. They are extremely hard, brittle, refractory ceramic materials with metallic elec ...
, zirconium carbide
Zirconium carbide () is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering.
Properties
It appears as a gray metallic powder with cubic crystal structure. It is ...
, tantalum nitride
Tantalum nitride (TaN) is a chemical compound, a nitride of tantalum. There are multiple phases of compounds, stoichimetrically from Ta2N to Ta3N5, including TaN.
As a thin film TaN find use as a diffusion barrier and insulating layer between cop ...
, and zirconium nitride.
Cathode heater
A ''cathode heater'' is a heated wire filament used to heat thecathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
in a vacuum tube
A vacuum tube, electron tube, thermionic valve (British usage), or tube (North America) is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. It ...
or cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a ...
. The cathode element has to achieve the required temperature in order for these tubes to function properly. This is why older electronics often need some time to "warm up" after being powered on; this phenomenon can still be observed in the cathode ray tubes of some modern televisions and computer monitor
A computer monitor is an output device that displays information in pictorial or textual form. A discrete monitor comprises a electronic visual display, visual display, support electronics, power supply, Housing (engineering), housing, electri ...
s. The cathode heats to a temperature that causes electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s to be 'boiled out' of its surface into the evacuated space in the tube, a process called thermionic emission
Thermionic emission is the liberation of charged particles from a hot electrode whose thermal energy gives some particles enough kinetic energy to escape the material's surface. The particles, sometimes called ''thermions'' in early literature, a ...
. The temperature required for modern oxide-coated cathodes is around .
The cathode is usually in the form of a long narrow sheet metal cylinder at the center of the tube. The heater consists of a fine wire or ribbon, made of a high resistance metal alloy like nichrome
Nichrome (also known as NiCr, nickel-chromium or chromium-nickel) is a family of alloys of nickel and chromium (and occasionally iron) commonly used as resistance wire, heating elements in devices like toasters, electrical kettles and space he ...
, similar to the heating element
A heating element is a device used for conversion of electric energy into heat, consisting of a heating resistor and accessories. Heat is generated by the passage of electric current through a resistor through a process known as Joule heating. He ...
in a toaster
A toaster is a small electric appliance that uses radiant heat to brown sliced bread into toast, the color caused by the Maillard reaction. It typically consists of one or more slots into which bread is inserted, and heating elements, o ...
but finer. It runs through the center of the cathode, often being coiled on tiny insulating supports or bent into hairpin-like shapes to give enough surface area to produce the required heat. Typical heaters have a ceramic coating on the wire. When it's bent sharply at the ends of the cathode sleeve, the wire is exposed.
The ends of the wire are electrically connected to two of the several pins protruding from the end of the tube. When current
Currents, Current or The Current may refer to:
Science and technology
* Current (fluid), the flow of a liquid or a gas
** Air current, a flow of air
** Ocean current, a current in the ocean
*** Rip current, a kind of water current
** Current (hydr ...
passes through the wire it becomes red hot, and the radiated heat strikes the inside surface of the cathode, heating it. The red or orange glow seen coming from operating vacuum tubes is produced by the heater.
There is not much room in the cathode, and the cathode is often built with the heater wire touching it. The inside of the cathode is insulated by a coating of alumina
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly ...
(aluminum oxide). This is not a very good insulator at high temperatures, therefore tubes have a rating for maximum voltage between cathode and heater, usually only 200 to 300 V.
Heaters require a low voltage, high current source of power. Miniature receiving tubes for line-operated equipment use on the order of 0.5 to 4 watts for heater power; high power tubes such as rectifiers or output tubes use on the order of 10 to 20 watts, and broadcast transmitter tubes might need a kilowatt or more to heat the cathode.Sōgo Okamura ''History of electron tubes'', IOS Press, 1994 , pp. 106, 109, 120, 144, 174
The voltage required is usually 5 or 6 volts AC. This is supplied by a separate 'heater winding' on the device's power supply transformer
In electrical engineering, a transformer is a passive component that transfers electrical energy from one electrical circuit to another circuit, or multiple Electrical network, circuits. A varying current in any coil of the transformer produces ...
that also supplies the higher voltages required by the tubes' plates and other electrodes. One approach used in transformerless line-operated radio and television receivers such as the All American Five
The term All American Five (abbreviated AA5) is a colloquial name for mass-produced, superheterodyne radio receivers that used five vacuum tubes in their design. These radio sets were designed to receive amplitude modulation (AM) broadcasts in the ...
is to connect all the tube heaters in series across the supply line. Since all the heaters are rated at the same current, they would share voltage according to their heater ratings.
Battery-operated radio sets used direct-current power for the heaters (commonly known as filaments), and tubes intended for battery
Battery or batterie most often refers to:
* Electric battery, a device that provides electrical power
* Battery (crime), a crime involving unlawful physical contact
Battery may also refer to:
Energy source
* Battery indicator, a device whic ...
sets were designed to use as little filament power as necessary, to economize on battery replacement. The final models of tube-equipped radio receivers were built with subminiature tubes using less than 50 mA for the heaters, but these types were developed at about the same time as transistors which replaced them.
Where leakage or stray fields from the heater circuit could potentially be coupled to the cathode, direct current is sometimes used for heater power. This eliminates a source of noise in sensitive audio or instrumentation circuits.
The majority of power required to operate low power tube equipment is consumed by the heaters. Transistors have no such power requirement, which is often a great advantage.
Failure modes
The emissive layers on coated cathodes degrade slowly with time, and much more quickly when the cathode is overloaded with too high current. The result is weakened emission and diminished power of the tubes, or in CRTs diminished brightness. The activated electrodes can be destroyed by contact withoxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
or other chemicals (e.g. aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, or silicate
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used ...
s), either present as residual gases, entering the tube via leaks, or released by outgassing
Outgassing (sometimes called offgassing, particularly when in reference to indoor air quality) is the release of a gas that was dissolved, trapped, frozen, or absorbed in some material. Outgassing can include sublimation and evaporation (whic ...
or migration from the construction elements. This results in diminished emissivity. This process is known as ''cathode poisoning''. High-reliability tubes had to be developed for the early Whirlwind
A whirlwind is a phenomenon in which a vortex of wind (a vertically oriented rotating column of air) forms due to instabilities and turbulence created by heating and flow ( current) gradients. Whirlwinds can vary in size and last from a cou ...
computer, with filaments free of traces of silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
.
Slow degradation of the emissive layer and sudden burning and interruption of the filament are two main failure mode
Failure causes are defects in design, process, quality, or part application, which are the underlying cause of a failure or which initiate a process which leads to failure. Where failure depends on the user of the product or process, then human er ...
s of vacuum tubes.
Transmitting tube hot cathode characteristics
See also
*Hot filament ionization gauge
The hot-filament ionization gauge, sometimes called a hot-filament gauge or hot-cathode gauge, is the most widely used low-pressure (vacuum) measuring device for the region from 10−3 to 10−10 Torr. It is a triode, with the filament being the ...
References
External links
John Harper (2003) ''Tubes 201 - How vacuum tubes really work'', John Harper's home page
* {{Thermionic valves Electrodes Gas discharge lamps Vacuum tubes Accelerator physics