Sulfur on:
[Wikipedia]
[Google]
[Amazon]
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a
here
The greatest commercial use of the element is the production of
 Sulfur forms several polyatomic molecules. The best-known allotrope is octasulfur, cyclo-S8. The point group of cyclo-S8 is D4d and its dipole moment is 0 D. Octasulfur is a soft, bright-yellow solid that is odorless. It melts at , and boils at . At , below its melting temperature, cyclo-octasulfur begins slowly changing from α-octasulfur to the β- polymorph. The structure of the S8 ring is virtually unchanged by this phase transition, which affects the intermolecular interactions. Cooling molten sulfur freezes at , as it predominantly consists of the β-S8 molecules. Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increased
Sulfur forms several polyatomic molecules. The best-known allotrope is octasulfur, cyclo-S8. The point group of cyclo-S8 is D4d and its dipole moment is 0 D. Octasulfur is a soft, bright-yellow solid that is odorless. It melts at , and boils at . At , below its melting temperature, cyclo-octasulfur begins slowly changing from α-octasulfur to the β- polymorph. The structure of the S8 ring is virtually unchanged by this phase transition, which affects the intermolecular interactions. Cooling molten sulfur freezes at , as it predominantly consists of the β-S8 molecules. Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increased
 Sulfur forms over 30 solid allotropes, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is of a deeper yellow than S8. HPLC analysis of "elemental sulfur" reveals an equilibrium mixture of mainly S8, but with S7 and small amounts of S6. Larger rings have been prepared, including S12 and S18.
Sulfur forms over 30 solid allotropes, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is of a deeper yellow than S8. HPLC analysis of "elemental sulfur" reveals an equilibrium mixture of mainly S8, but with S7 and small amounts of S6. Larger rings have been prepared, including S12 and S18.
 32S is created inside massive stars, at a depth where the temperature exceeds 2.5 billion K, by the fusion of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the alpha process that produces elements in abundance, sulfur is the 10th most common element in the universe.
Sulfur, usually as sulfide, is present in many types of meteorites. Ordinary chondrites contain on average 2.1% sulfur, and carbonaceous chondrites may contain as much as 6.6%. It is normally present as troilite (FeS), but there are exceptions, with carbonaceous chondrites containing free sulfur, sulfates and other sulfur compounds. The distinctive colors of
32S is created inside massive stars, at a depth where the temperature exceeds 2.5 billion K, by the fusion of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the alpha process that produces elements in abundance, sulfur is the 10th most common element in the universe.
Sulfur, usually as sulfide, is present in many types of meteorites. Ordinary chondrites contain on average 2.1% sulfur, and carbonaceous chondrites may contain as much as 6.6%. It is normally present as troilite (FeS), but there are exceptions, with carbonaceous chondrites containing free sulfur, sulfates and other sulfur compounds. The distinctive colors of
File:L-Cystein - L-Cysteine.svg , (''L'')- cysteine, an amino acid containing a thiol group
File:Methionin - Methionine.svg, Methionine, an amino acid containing a thioether
File:Thiamin.svg,
Some of the main classes of sulfur-containing organic compounds include the following:
* Thiols or mercaptans (so called because they capture mercury as chelators) are the sulfur analogs of
 According to the Ebers Papyrus, a sulfur ointment was used in ancient
According to the Ebers Papyrus, a sulfur ointment was used in ancient
 Sulfur appears in a column of fixed (non-acidic) alkali in a chemical table of 1718. Antoine Lavoisier used sulfur in combustion experiments, writing of some of these in 1777.
Sulfur deposits in
Sulfur appears in a column of fixed (non-acidic) alkali in a chemical table of 1718. Antoine Lavoisier used sulfur in combustion experiments, writing of some of these in 1777.
Sulfur deposits in
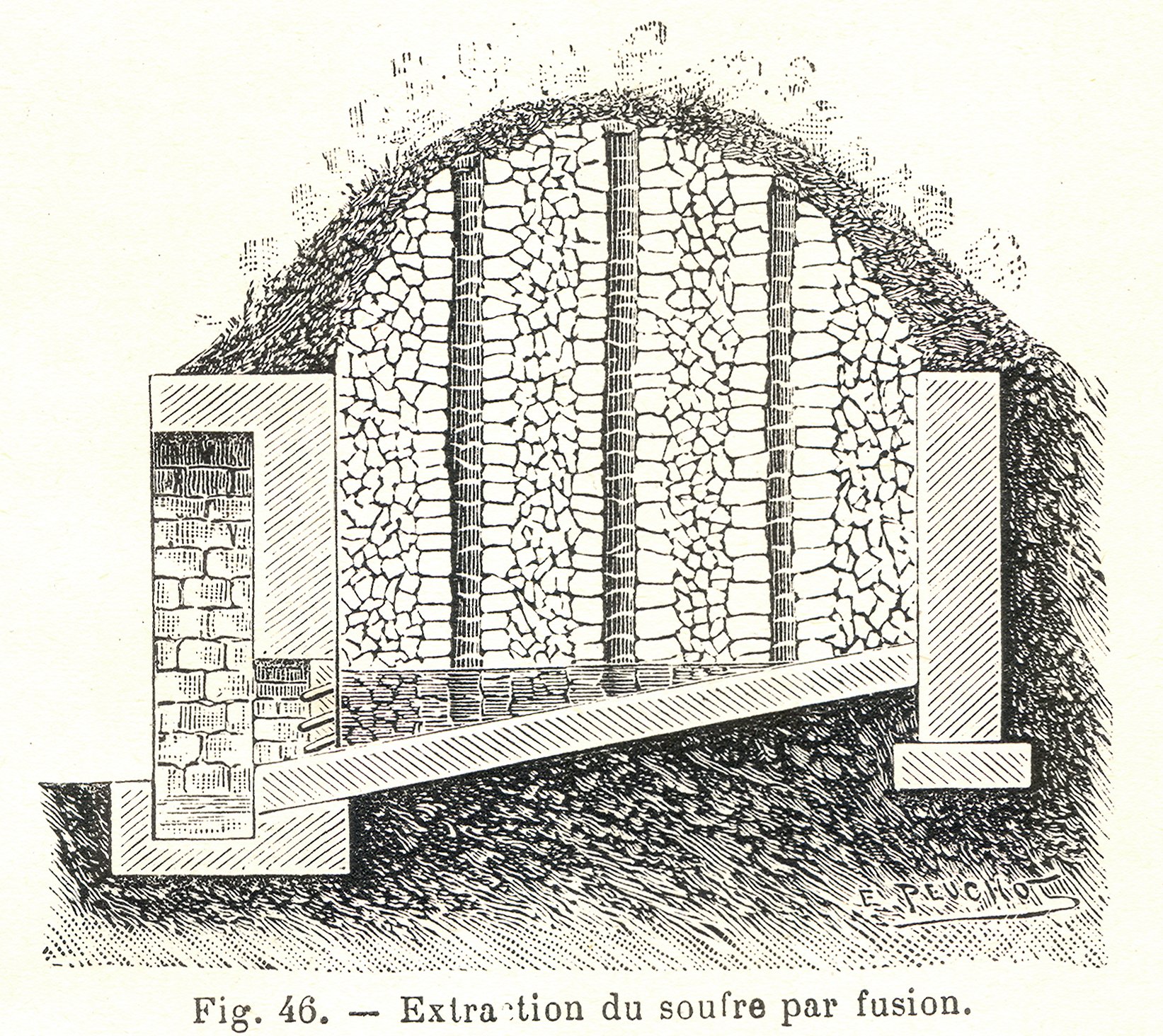
 Sulfur may be found by itself and historically was usually obtained in this form; pyrite has also been a source of sulfur. In volcanic regions in
Sulfur may be found by itself and historically was usually obtained in this form; pyrite has also been a source of sulfur. In volcanic regions in  Since then, sulfur has typically been produced from petroleum, natural gas, and related fossil resources, from which it is obtained mainly as hydrogen sulfide. Organosulfur compounds, undesirable impurities in petroleum, may be upgraded by subjecting them to hydrodesulfurization, which cleaves the C–S bonds:
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by the Claus process, which entails oxidation of some hydrogen sulfide to sulfur dioxide and then the comproportionation of the two:
Since then, sulfur has typically been produced from petroleum, natural gas, and related fossil resources, from which it is obtained mainly as hydrogen sulfide. Organosulfur compounds, undesirable impurities in petroleum, may be upgraded by subjecting them to hydrodesulfurization, which cleaves the C–S bonds:
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by the Claus process, which entails oxidation of some hydrogen sulfide to sulfur dioxide and then the comproportionation of the two:
 Due to the high sulfur content of the Athabasca Oil Sands, stockpiles of elemental sulfur from this process exist throughout
Due to the high sulfur content of the Athabasca Oil Sands, stockpiles of elemental sulfur from this process exist throughout
 In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
 Elemental sulfur is one of the oldest fungicides and
Elemental sulfur is one of the oldest fungicides and
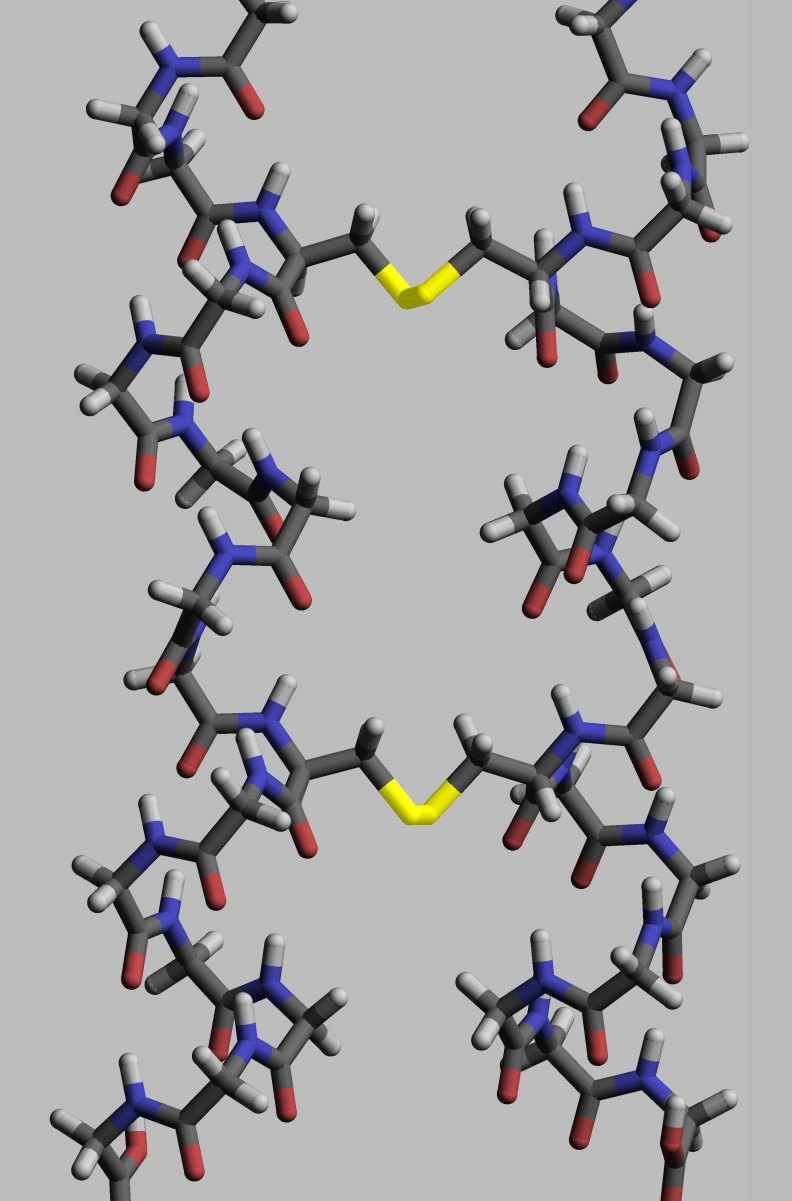 The functionality of a given protein is heavily dependent on its structure. Proteins reach this structure through the process of protein folding, which is facilitated by a variety of intra- and inter-molecular bonds. While much of the folding is driven by the formation of hydrogen bonds, covalent bonding of cysteine residues into disulfide bridges imposes constraints that stabilize particular conformations while preventing others from forming. As the bond energy of a covalent disulfide bridge is higher than the energy of a coordinate bond or hydrophobic interaction, greater numbers of disulfide bridges lead to higher energies required for protein denaturation. Disulfide bonds often serve to stabilize protein structures in the more oxidizing conditions of the extracellular environment. Within the
The functionality of a given protein is heavily dependent on its structure. Proteins reach this structure through the process of protein folding, which is facilitated by a variety of intra- and inter-molecular bonds. While much of the folding is driven by the formation of hydrogen bonds, covalent bonding of cysteine residues into disulfide bridges imposes constraints that stabilize particular conformations while preventing others from forming. As the bond energy of a covalent disulfide bridge is higher than the energy of a coordinate bond or hydrophobic interaction, greater numbers of disulfide bridges lead to higher energies required for protein denaturation. Disulfide bonds often serve to stabilize protein structures in the more oxidizing conditions of the extracellular environment. Within the
 Though elemental sulfur is only minimally absorbed through the skin and is of low toxicity to humans, inhalation of sulfur dust or contact with eyes or skin may cause irritation. Excessive ingestion of sulfur can cause a burning sensation or diarrhea, and cases of life-threatening metabolic acidosis have been reported after patients deliberately consumed sulfur as a folk remedy.
Though elemental sulfur is only minimally absorbed through the skin and is of low toxicity to humans, inhalation of sulfur dust or contact with eyes or skin may cause irritation. Excessive ingestion of sulfur can cause a burning sensation or diarrhea, and cases of life-threatening metabolic acidosis have been reported after patients deliberately consumed sulfur as a folk remedy.
Sulfur
at '' The Periodic Table of Videos'' (University of Nottingham)
Atomic Data for Sulfur
NIST Physical Measurement Laboratory
Sulfur phase diagram
, Introduction to Chemistry for Ages 13–17
* ttps://extoxnet.orst.edu/pips/sulfur.htm Sulfur and its use as a pesticidebr>The Sulphur InstituteNutrient Stewardship and The Sulphur Institute
{{Authority control Chemical elements Chalcogens Reactive nonmetals Polyatomic nonmetals Agricultural chemicals Anti-acne preparations Dietary minerals Industrial minerals Inorganic polymers Native element minerals Orthorhombic minerals Minerals in space group 70 Pyrotechnic fuels Chemical elements with primitive orthorhombic structure
chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature.
Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India
Anatomically modern humans first arrived on the Indian subcontinent between 73,000 and 55,000 years ago. The earliest known human remains in South Asia date to 30,000 years ago. Sedentism, Sedentariness began in South Asia around 7000 BCE; ...
, ancient Greece
Ancient Greece () was a northeastern Mediterranean civilization, existing from the Greek Dark Ages of the 12th–9th centuries BC to the end of classical antiquity (), that comprised a loose collection of culturally and linguistically r ...
, China
China, officially the People's Republic of China (PRC), is a country in East Asia. With population of China, a population exceeding 1.4 billion, it is the list of countries by population (United Nations), second-most populous country after ...
, and ancient Egypt
Ancient Egypt () was a cradle of civilization concentrated along the lower reaches of the Nile River in Northeast Africa. It emerged from prehistoric Egypt around 3150BC (according to conventional Egyptian chronology), when Upper and Lower E ...
. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
.. Downloahere
The greatest commercial use of the element is the production of
sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
for sulfate and phosphate fertilizers, and other chemical processes. Sulfur is used in matches, insecticide
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, i ...
s, and fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, ...
s. Many sulfur compounds are odoriferous, and the smells of odorized natural gas, skunk scent, bad breath, grapefruit, and garlic are due to organosulfur compounds. Hydrogen sulfide gives the characteristic odor to rotting eggs and other biological processes.
Sulfur is an essential element for all life, almost always in the form of organosulfur compounds or metal sulfides. Amino acids (two proteinogenic: cysteine and methionine, and many other non-coded: cystine, taurine, etc.) and two vitamins ( biotin and thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin – an Nutrient#Micronutrients, essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosp ...
) are organosulfur compounds crucial for life. Many cofactors also contain sulfur, including glutathione, and iron–sulfur proteins. Disulfides, S–S bonds, confer mechanical strength and insolubility of the (among others) protein keratin, found in outer skin, hair, and feathers. Sulfur is one of the core chemical elements needed for biochemical
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, ...
functioning and is an elemental macronutrient for all living organisms.
Characteristics
Physical properties
 Sulfur forms several polyatomic molecules. The best-known allotrope is octasulfur, cyclo-S8. The point group of cyclo-S8 is D4d and its dipole moment is 0 D. Octasulfur is a soft, bright-yellow solid that is odorless. It melts at , and boils at . At , below its melting temperature, cyclo-octasulfur begins slowly changing from α-octasulfur to the β- polymorph. The structure of the S8 ring is virtually unchanged by this phase transition, which affects the intermolecular interactions. Cooling molten sulfur freezes at , as it predominantly consists of the β-S8 molecules. Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increased
Sulfur forms several polyatomic molecules. The best-known allotrope is octasulfur, cyclo-S8. The point group of cyclo-S8 is D4d and its dipole moment is 0 D. Octasulfur is a soft, bright-yellow solid that is odorless. It melts at , and boils at . At , below its melting temperature, cyclo-octasulfur begins slowly changing from α-octasulfur to the β- polymorph. The structure of the S8 ring is virtually unchanged by this phase transition, which affects the intermolecular interactions. Cooling molten sulfur freezes at , as it predominantly consists of the β-S8 molecules. Between its melting and boiling temperatures, octasulfur changes its allotrope again, turning from β-octasulfur to γ-sulfur, again accompanied by a lower density but increased viscosity
Viscosity is a measure of a fluid's rate-dependent drag (physics), resistance to a change in shape or to movement of its neighboring portions relative to one another. For liquids, it corresponds to the informal concept of ''thickness''; for e ...
due to the formation of polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
s. At higher temperatures, the viscosity decreases as depolymerization occurs. Molten sulfur assumes a dark red color above . The density of sulfur is about 2 g/cm3, depending on the allotrope; all of the stable allotropes are excellent electrical insulators.
The sublimation of sulfur becomes noticeable more or less between and , and occurs readily in boiling water at .
Sulfur is insoluble in water but soluble in carbon disulfide and, to a lesser extent, in other nonpolar organic solvents, such as benzene and toluene.
Chemical properties
Under normal conditions, sulfur hydrolyzes very slowly to mainly form hydrogen sulfide andsulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
:
The reaction involves adsorption of protons onto clusters, followed by disproportionation into the reaction products.
The second, fourth and sixth ionization energies of sulfur are 2252 kJ/mol, 4556 kJ/mol and 8495.8 kJ/mol, respectively. The composition of reaction products of sulfur with oxidants (and its oxidation state) depends on whether releasing of reaction energy overcomes these thresholds. Applying catalysts
Catalysis () is the increase in reaction rate, rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst ...
and/or supply of external energy may vary sulfur's oxidation state and the composition of reaction products. While reaction between sulfur and oxygen under normal conditions gives sulfur dioxide (oxidation state +4), formation of sulfur trioxide (oxidation state +6) requires a temperature of and presence of a catalyst.
In reactions with elements of lesser electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
, it reacts as an oxidant and forms sulfides, where it has oxidation state −2.
Sulfur reacts with nearly all other elements except noble gases, even with the notoriously unreactive metal iridium (yielding iridium disulfide). Some of those reactions require elevated temperatures.
Allotropes
 Sulfur forms over 30 solid allotropes, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is of a deeper yellow than S8. HPLC analysis of "elemental sulfur" reveals an equilibrium mixture of mainly S8, but with S7 and small amounts of S6. Larger rings have been prepared, including S12 and S18.
Sulfur forms over 30 solid allotropes, more than any other element. Besides S8, several other rings are known. Removing one atom from the crown gives S7, which is of a deeper yellow than S8. HPLC analysis of "elemental sulfur" reveals an equilibrium mixture of mainly S8, but with S7 and small amounts of S6. Larger rings have been prepared, including S12 and S18.
Amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
or "plastic" sulfur is produced by rapid cooling of molten sulfur—for example, by pouring it into cold water. X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
studies show that the amorphous form may have a helical structure with eight atoms per turn. The long coiled polymeric molecules make the brownish substance elastic
Elastic is a word often used to describe or identify certain types of elastomer, Elastic (notion), elastic used in garments or stretch fabric, stretchable fabrics.
Elastic may also refer to:
Alternative name
* Rubber band, ring-shaped band of rub ...
, and in bulk it has the feel of crude rubber. This form is metastable at room temperature and gradually reverts to the crystalline molecular allotrope, which is no longer elastic. This process happens over a matter of hours to days, but can be rapidly catalyzed.
Isotopes
Sulfur has 23 knownisotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s, four of which are stable: 32S (), 33S (), 34S (), and 36S (). Other than 35S, with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 87 days, the radioactive isotopes of sulfur have half-lives less than 3 hours.
The preponderance of 32S is explained by its production in the alpha process (one of the main classes of nuclear fusion reactions) in exploding stars. Other stable sulfur isotopes are produced in the bypass processes related with 34Ar, and their composition depends on a type of a stellar explosion. For example, proportionally more 33S comes from novae than from supernovae.
On the planet Earth the sulfur isotopic composition was determined by the Sun. Though it was assumed that the distribution of different sulfur isotopes would be more or less equal, it has been found that proportions of the two most abundant sulfur isotopes 32S and 34S varies in different samples. Assaying of the isotope ratio ( δ34S) in the samples suggests their chemical history, and with support of other methods, it allows to age-date the samples, estimate temperature of equilibrium between ore and water, determine pH and oxygen fugacity, identify the activity of sulfate-reducing bacteria in the time of formation of the sample, or suggest the main sources of sulfur in ecosystems. However, there are ongoing discussions over the real reason for the δ34S shifts, biological activity or postdeposit alteration.
For example, when sulfide minerals are precipitated, isotopic equilibration among solids and liquid may cause small differences in the δ34S values of co-genetic minerals. The differences between minerals can be used to estimate the temperature of equilibration. The δ13C and δ34S of coexisting carbonate minerals and sulfides can be used to determine the pH and oxygen fugacity of the ore-bearing fluid during ore formation.
Scientists measure the sulfur isotopes of minerals in rocks and sediments to study the redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
conditions in past oceans. Sulfate-reducing bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as termina ...
in marine sediment fractionate sulfur isotopes as they take in sulfate and produce sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
. Prior to the 2010s, it was thought that sulfate reduction could fractionate sulfur isotopes up to 46 permil and fractionation larger than 46 permil recorded in sediments must be due to disproportionation of sulfur compounds in the sediment. This view has changed since the 2010s as experiments showed that sulfate-reducing bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as termina ...
can fractionate to 66 permil. As substrates for disproportionation are limited by the product of sulfate reduction, the isotopic effect of disproportionation should be less than 16 permil in most sedimentary settings.
In forest
A forest is an ecosystem characterized by a dense ecological community, community of trees. Hundreds of definitions of forest are used throughout the world, incorporating factors such as tree density, tree height, land use, legal standing, ...
ecosystems, sulfate is derived mostly from the atmosphere; weathering of ore minerals and evaporites contribute some sulfur. Sulfur with a distinctive isotopic composition has been used to identify pollution sources, and enriched sulfur has been added as a tracer in hydrologic studies. Differences in the natural abundances can be used in systems where there is sufficient variation in the 34S of ecosystem components. Rocky Mountain lakes thought to be dominated by atmospheric sources of sulfate have been found to have measurably different 34S values than lakes believed to be dominated by watershed sources of sulfate.
The radioactive 35S is formed in cosmic ray spallation of the atmospheric 40Ar. This fact may be used to verify the presence of recent (up to 1 year) atmospheric sediments in various materials. This isotope may be obtained artificially by different ways. In practice, the reaction 35Cl + n → 35S + p is used by irradiating potassium chloride with neutrons. The isotope 35S is used in various sulfur-containing compounds as a radioactive tracer for many biological studies, for example, the Hershey-Chase experiment.
Because of the weak beta activity of 35S, its compounds are relatively safe as long as they are not ingested or absorbed by the body.
Natural occurrence
 32S is created inside massive stars, at a depth where the temperature exceeds 2.5 billion K, by the fusion of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the alpha process that produces elements in abundance, sulfur is the 10th most common element in the universe.
Sulfur, usually as sulfide, is present in many types of meteorites. Ordinary chondrites contain on average 2.1% sulfur, and carbonaceous chondrites may contain as much as 6.6%. It is normally present as troilite (FeS), but there are exceptions, with carbonaceous chondrites containing free sulfur, sulfates and other sulfur compounds. The distinctive colors of
32S is created inside massive stars, at a depth where the temperature exceeds 2.5 billion K, by the fusion of one nucleus of silicon plus one nucleus of helium. As this nuclear reaction is part of the alpha process that produces elements in abundance, sulfur is the 10th most common element in the universe.
Sulfur, usually as sulfide, is present in many types of meteorites. Ordinary chondrites contain on average 2.1% sulfur, and carbonaceous chondrites may contain as much as 6.6%. It is normally present as troilite (FeS), but there are exceptions, with carbonaceous chondrites containing free sulfur, sulfates and other sulfur compounds. The distinctive colors of Jupiter
Jupiter is the fifth planet from the Sun and the List of Solar System objects by size, largest in the Solar System. It is a gas giant with a Jupiter mass, mass more than 2.5 times that of all the other planets in the Solar System combined a ...
's volcanic moon Io are attributed to various forms of molten, solid, and gaseous sulfur. In July 2024, elemental sulfur was accidentally discovered to exist on Mars after the Curiosity rover
''Curiosity'' is a car-sized Mars rover Space exploration, exploring Gale (crater), Gale crater and Mount Sharp on Mars as part of NASA's Mars Science Laboratory (MSL) mission. ''Curiosity'' was launched from Cape Canaveral Space Force Station ...
drove over and crushed a rock, revealing sulfur crystals inside it.
Sulfur is the fifth most common element by mass in the Earth. Elemental sulfur can be found near hot springs and volcanic regions in many parts of the world, especially along the Pacific Ring of Fire; such volcanic deposits are mined in Indonesia, Chile, and Japan. These deposits are polycrystalline, with the largest documented single crystal measuring . Historically, Sicily
Sicily (Italian language, Italian and ), officially the Sicilian Region (), is an island in the central Mediterranean Sea, south of the Italian Peninsula in continental Europe and is one of the 20 regions of Italy, regions of Italy. With 4. ...
was a major source of sulfur in the Industrial Revolution
The Industrial Revolution, sometimes divided into the First Industrial Revolution and Second Industrial Revolution, was a transitional period of the global economy toward more widespread, efficient and stable manufacturing processes, succee ...
. Lakes of molten sulfur up to about in diameter have been found on the sea floor, associated with submarine volcanoes, at depths where the boiling point of water is higher than the melting point of sulfur.
Native sulfur is synthesized by anaerobic bacteria acting on sulfate minerals such as gypsum in salt domes. Significant deposits in salt domes occur along the coast of the Gulf of Mexico
The Gulf of Mexico () is an oceanic basin and a marginal sea of the Atlantic Ocean, mostly surrounded by the North American continent. It is bounded on the northeast, north, and northwest by the Gulf Coast of the United States; on the southw ...
, and in evaporites in eastern Europe and western Asia. Native sulfur may be produced by geological processes alone. Fossil-based sulfur deposits from salt domes were once the basis for commercial production in the United States, Russia, Turkmenistan, and Ukraine. Such sources have become of secondary commercial importance, and most are no longer worked but commercial production is still carried out in the Osiek mine in Poland.
Common naturally occurring sulfur compounds include the sulfide minerals, such as pyrite (iron sulfide), cinnabar (mercury sulfide), galena (lead sulfide), sphalerite
Sphalerite is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimentary exhalative, Carbonate-hoste ...
(zinc sulfide), and stibnite (antimony sulfide); and the sulfate minerals, such as gypsum (calcium sulfate), alunite (potassium aluminium sulfate), and barite (barium sulfate). On Earth, just as upon Jupiter's moon Io, elemental sulfur occurs naturally in volcanic emissions, including emissions from hydrothermal vents.
The main industrial source of sulfur has become petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
and natural gas.
Compounds
Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except thenoble gas
The noble gases (historically the inert gases, sometimes referred to as aerogens) are the members of Group (periodic table), group 18 of the periodic table: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn) and, in some ...
es.
Electron transfer reactions
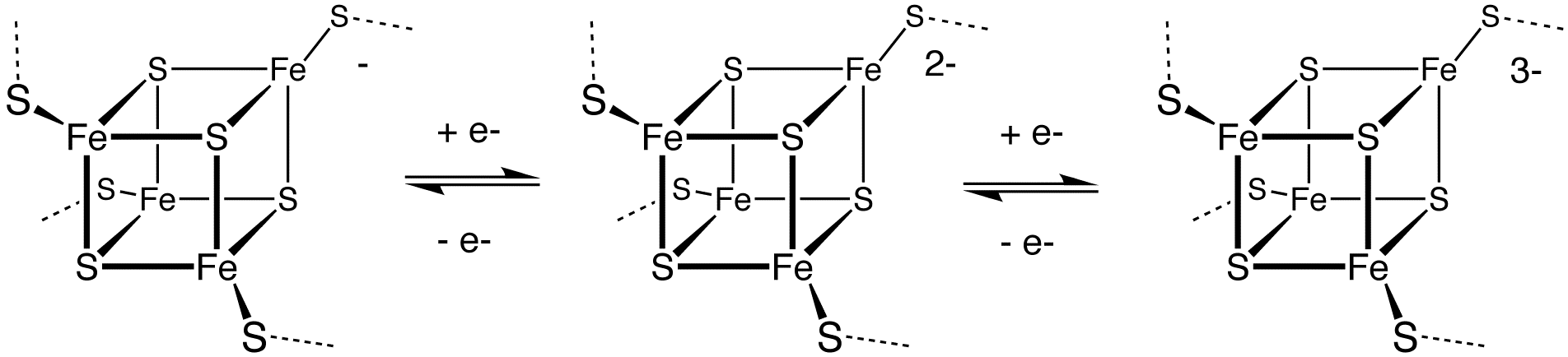
Sulfur polycations, , and are produced when sulfur is reacted with oxidizing agents in a strongly acidic solution. The colored solutions produced by dissolving sulfur in oleum were first reported as early as 1804 by C. F. Bucholz, but the cause of the color and the structure of the polycations involved was only determined in the late 1960s. is deep blue, is yellow and is red. Reduction of sulfur gives various polysulfides with the formula , many of which have been obtained in crystalline form. Illustrative is the production of sodium tetrasulfide: Some of these dianions dissociate to give radical anions. For instance, gives the blue color of the rock lapis lazuli. This reaction highlights a distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains). Protonation of these polysulfide anions produces the polysulfanes, H2S''x'', where ''x'' = 2, 3, and 4. Ultimately, reduction of sulfur produces sulfide salts: The interconversion of these species is exploited in the sodium–sulfur battery.Hydrogenation
Treatment of sulfur with hydrogen gives hydrogen sulfide. When dissolved in water, hydrogen sulfide is mildly acidic: Hydrogen sulfide gas and the hydrosulfide anion are extremely toxic to mammals, due to their inhibition of the oxygen-carrying capacity ofhemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
and certain cytochromes in a manner analogous to cyanide and azide (see below, under ''precautions'').
Combustion
The two principal sulfur oxides are obtained by burning sulfur: Many other sulfur oxides are observed including the sulfur-rich oxides include sulfur monoxide, disulfur monoxide, disulfur dioxides, and higher oxides containing peroxo groups.Halogenation
Sulfur reacts withfluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extre ...
to give the highly reactive sulfur tetrafluoride and the highly inert sulfur hexafluoride. Whereas fluorine gives S(IV) and S(VI) compounds, chlorine gives S(II) and S(I) derivatives. Thus, sulfur dichloride, disulfur dichloride, and higher chlorosulfanes arise from the chlorination of sulfur. Sulfuryl chloride and chlorosulfuric acid are derivatives of sulfuric acid; thionyl chloride (SOCl2) is a common reagent in organic synthesis. Bromine also oxidizes sulfur to form sulfur dibromide and disulfur dibromide.
Pseudohalides
Sulfur oxidizes cyanide and sulfite to give thiocyanate andthiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
, respectively.
Metal sulfides
Sulfur reacts with many metals. Electropositive metals give polysulfide salts. Copper, zinc, and silver are attacked by sulfur; see tarnishing. Although many metal sulfides are known, most are prepared by high temperature reactions of the elements. Geoscientists also study the isotopes of metal sulfides in rocks and sediment to study environmental conditions in the Earth's past.Organic compounds
Thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin – an Nutrient#Micronutrients, essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosp ...
or vitamin B1
File:Biotin_structure.svg, Biotin or vitamin B7
File:Penicillin core.svg, Penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
, an antibiotic ("R" is the variable group)
File:Allicin skeletal.svg, Allicin, a chemical compound in garlic
File:Diphenyl disulfide.svg, Diphenyl disulfide, a representative disulfide
File:Dibenzothiophen - Dibenzothiophene.svg, Dibenzothiophene, a component of crude oil
File:Perfluorooctanesulfonic acid structure.svg, Perfluorooctanesulfonic acid (PFOS), a surfactant
alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s; treatment of thiols with base gives thiolate ions.
* Thioethers are the sulfur analogs of ethers.
* Sulfonium ions have three groups attached to a cationic sulfur center. Dimethylsulfoniopropionate (DMSP) is one such compound, important in the marine organic sulfur cycle.
* Sulfoxides and sulfones are thioethers with one and two oxygen atoms attached to the sulfur atom, respectively. The simplest sulfoxide, dimethyl sulfoxide, is a common solvent; a common sulfone is sulfolane.
* Sulfonic acids are used in many detergents.
Compounds with carbon–sulfur multiple bonds are uncommon, an exception being carbon disulfide, a volatile colorless liquid that is structurally similar to carbon dioxide. It is used as a reagent to make the polymer rayon and many organosulfur compounds. Unlike carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
, carbon monosulfide is stable only as an extremely dilute gas, found between solar systems.
Organosulfur compounds are responsible for some of the unpleasant odors of decaying organic matter. They are widely known as the odorant in domestic natural gas, garlic odor, and skunk spray, as well as a component of bad breath odor. Not all organic sulfur compounds smell unpleasant at all concentrations: the sulfur-containing monoterpenoid grapefruit mercaptan in small concentrations is the characteristic scent of grapefruit, but has a generic thiol odor at larger concentrations. Sulfur mustard, a potent vesicant, was used in World War I as a disabling agent.
Sulfur–sulfur bonds are a structural component used to stiffen rubber, similar to the disulfide bridges that rigidify proteins (see biological below). In the most common type of industrial "curing" or hardening and strengthening of natural rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds.
Types of polyisoprene ...
, elemental sulfur is heated with the rubber to the point that chemical reactions form disulfide bridges between isoprene units of the polymer. This process, patented in 1843, made rubber a major industrial product, especially in automobile tires. Because of the heat and sulfur, the process was named vulcanization, after the Roman god of the forge and volcanism.
History
Antiquity
 According to the Ebers Papyrus, a sulfur ointment was used in ancient
According to the Ebers Papyrus, a sulfur ointment was used in ancient Egypt
Egypt ( , ), officially the Arab Republic of Egypt, is a country spanning the Northeast Africa, northeast corner of Africa and Western Asia, southwest corner of Asia via the Sinai Peninsula. It is bordered by the Mediterranean Sea to northe ...
to treat granular eyelids. Sulfur was used for fumigation in preclassical Greece
Greece, officially the Hellenic Republic, is a country in Southeast Europe. Located on the southern tip of the Balkan peninsula, it shares land borders with Albania to the northwest, North Macedonia and Bulgaria to the north, and Turkey to th ...
; this is mentioned in the '' Odyssey''. Pliny the Elder
Gaius Plinius Secundus (AD 23/24 79), known in English as Pliny the Elder ( ), was a Roman Empire, Roman author, Natural history, naturalist, and naval and army commander of the early Roman Empire, and a friend of the Roman emperor, emperor Vesp ...
discusses sulfur his '' Natural History'', saying that its best-known source is the island of Melos
Milos or Melos (; , ; ) is a volcanic Greek island in the Aegean Sea, just north of the Sea of Crete. It is the southwestern-most island of the Cyclades group.
The ''Venus de Milo'' (now in the Louvre), the '' Poseidon of Melos'' (now in the ...
. He mentions its use for fumigation, medicine, and bleaching cloth.
A natural form of sulfur known as was known in China since the 6th century BC and found in Hanzhong. By the 3rd century, the Chinese had discovered that sulfur could be extracted from pyrite. Chinese Daoists were interested in sulfur's flammability and its reactivity with certain metals, yet its earliest practical uses were found in traditional Chinese medicine
Traditional Chinese medicine (TCM) is an alternative medicine, alternative medical practice drawn from traditional medicine in China. A large share of its claims are pseudoscientific, with the majority of treatments having no robust evidence ...
. The '' Wujing Zongyao'' of 1044 AD described formulas for Chinese black powder
Gunpowder, also commonly known as black powder to distinguish it from modern smokeless powder, is the earliest known chemical explosive. It consists of a mixture of sulfur, charcoal (which is mostly carbon), and potassium nitrate, potassium ni ...
, which is a mixture of potassium nitrate, charcoal, and sulfur.
English translations of the Christian Bible commonly referred to burning sulfur as "brimstone", giving rise to the term " fire-and-brimstone" sermon
A sermon is a religious discourse or oration by a preacher, usually a member of clergy. Sermons address a scriptural, theological, or moral topic, usually expounding on a type of belief, law, or behavior within both past and present context ...
s, in which listeners are reminded of the fate of eternal damnation that await the unbelieving and unrepentant. Hell is implied to smell of sulfur.
Indian alchemists, practitioners of the "science of chemicals" (), wrote extensively about the use of sulfur in alchemical operations with mercury, from the eighth century AD onwards. In the tradition, sulfur is called "the smelly" (, ).
Early Europe
Europe is a continent located entirely in the Northern Hemisphere and mostly in the Eastern Hemisphere. It is bordered by the Arctic Ocean to the north, the Atlantic Ocean to the west, the Mediterranean Sea to the south, and Asia to the east ...
an alchemists gave sulfur an alchemical symbol of a triangle atop a cross (🜍). The variation known as brimstone has a symbol combining a two-barred cross atop a lemniscate (🜏). In traditional skin treatment, elemental sulfur was used (mainly in creams) to alleviate such conditions as scabies, ringworm
Dermatophytosis, also known as tinea and ringworm, is a mycosis, fungal infection of the skin (a dermatomycosis), that may affect skin, hair, and nails. Typically it results in a red, itchy, scaly, circular rash. Hair loss may occur in the a ...
, psoriasis, eczema, and acne
Acne ( ), also known as ''acne vulgaris'', is a long-term Cutaneous condition, skin condition that occurs when Keratinocyte, dead skin cells and Sebum, oil from the skin clog hair follicles. Typical features of the condition include comedo, ...
. The mechanism of action is unknown—though elemental sulfur does oxidize slowly to sulfurous acid, a mild reducing and antibacterial agent.
Modern times
 Sulfur appears in a column of fixed (non-acidic) alkali in a chemical table of 1718. Antoine Lavoisier used sulfur in combustion experiments, writing of some of these in 1777.
Sulfur deposits in
Sulfur appears in a column of fixed (non-acidic) alkali in a chemical table of 1718. Antoine Lavoisier used sulfur in combustion experiments, writing of some of these in 1777.
Sulfur deposits in Sicily
Sicily (Italian language, Italian and ), officially the Sicilian Region (), is an island in the central Mediterranean Sea, south of the Italian Peninsula in continental Europe and is one of the 20 regions of Italy, regions of Italy. With 4. ...
were the dominant source for more than a century. By the late 18th century, about 2,000 tonnes per year of sulfur were imported into Marseille
Marseille (; ; see #Name, below) is a city in southern France, the Prefectures in France, prefecture of the Departments of France, department of Bouches-du-Rhône and of the Provence-Alpes-Côte d'Azur Regions of France, region. Situated in the ...
, France, for the production of sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
for use in the Leblanc process. In industrializing Britain, with the repeal of tariff
A tariff or import tax is a duty (tax), duty imposed by a national Government, government, customs territory, or supranational union on imports of goods and is paid by the importer. Exceptionally, an export tax may be levied on exports of goods ...
s on salt in 1824, demand for sulfur from Sicily surged. The increasing British control and exploitation of the mining, refining, and transportation of sulfur, coupled with the failure of this lucrative export to transform Sicily's backward and impoverished economy, led to the Sulfur Crisis of 1840, when King Ferdinand II gave a monopoly of the sulfur industry to a French firm, violating an earlier 1816 trade agreement with Britain. A peaceful solution was eventually negotiated by France.
In 1867, elemental sulfur was discovered in underground deposits in Louisiana
Louisiana ( ; ; ) is a state in the Deep South and South Central regions of the United States. It borders Texas to the west, Arkansas to the north, and Mississippi to the east. Of the 50 U.S. states, it ranks 31st in area and 25 ...
and Texas
Texas ( , ; or ) is the most populous U.S. state, state in the South Central United States, South Central region of the United States. It borders Louisiana to the east, Arkansas to the northeast, Oklahoma to the north, New Mexico to the we ...
. The highly successful Frasch process was developed to extract this resource.
In the late 18th century, furniture
Furniture refers to objects intended to support various human activities such as seating (e.g., Stool (seat), stools, chairs, and sofas), eating (table (furniture), tables), storing items, working, and sleeping (e.g., beds and hammocks). Furnitur ...
makers used molten sulfur to produce decorative inlays. Molten sulfur is sometimes still used for setting steel bolts into drilled concrete holes where high shock resistance is desired for floor-mounted equipment attachment points. Pure powdered sulfur was used as a medicinal tonic and laxative.
Since the advent of the contact process, the majority of sulfur is used to make sulfuric acid for a wide range of uses, particularly fertilizer.
In recent times, the main source of sulfur has become petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
and natural gas. This is due to the requirement to remove sulfur from fuels in order to prevent acid rain
Acid rain is rain or any other form of Precipitation (meteorology), precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists b ...
, and has resulted in a surplus of sulfur.
Spelling and etymology
''Sulfur'' is derived from the Latin word ', which was Hellenized to ' in the erroneous belief that the Latin word came from Greek. This spelling was later reinterpreted as representing an /f/ sound and resulted in the spelling ', which appears in Latin toward the end of the Classical period. The true Ancient Greek word for sulfur, , ''theîon'' (from earlier , ''théeion''), is the source of the international chemical prefix '' thio-''. The Modern Standard Greek word for sulfur is θείο, ''theío''. In 12th-century Anglo-French, it was '. In the 14th century, the erroneously Hellenized Latin ' was restored in Middle English '. By the 15th century, both full Latin spelling variants ''sulfur'' and ''sulphur'' became common in English. The parallel ''f~ph'' spellings continued in Britain until the 19th century, when the word was standardized as ''sulphur''. On the other hand, ''sulfur'' was the form eventually chosen in the United States, though multiple place names (such as White Sulphur Springs) use ''-ph-''. Canada uses both spellings.IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
adopted the spelling ''sulfur'' in 1990 as did the Nomenclature Committee of the Royal Society of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the ...
in 1992, restoring the spelling ''sulfur'' to Britain. Oxford Dictionaries note that "in chemistry and other technical uses ... the ''-f-'' spelling is now the standard form for this and related words in British as well as US contexts, and is increasingly used in general contexts as well."
Production

 Sulfur may be found by itself and historically was usually obtained in this form; pyrite has also been a source of sulfur. In volcanic regions in
Sulfur may be found by itself and historically was usually obtained in this form; pyrite has also been a source of sulfur. In volcanic regions in Sicily
Sicily (Italian language, Italian and ), officially the Sicilian Region (), is an island in the central Mediterranean Sea, south of the Italian Peninsula in continental Europe and is one of the 20 regions of Italy, regions of Italy. With 4. ...
, in ancient times, it was found on the surface of the Earth, and the " Sicilian process" was used: sulfur deposits were piled and stacked in brick kilns built on sloping hillsides, with airspaces between them. Then, some sulfur was pulverized, spread over the stacked ore and ignited, causing the free sulfur to melt down the hills. Eventually the surface-borne deposits played out, and miners excavated veins that ultimately dotted the Sicilian landscape with labyrinthine mines. Mining was unmechanized and labor-intensive, with pickmen freeing the ore from the rock, and mine-boys or '' carusi'' carrying baskets of ore to the surface, often through a mile or more of tunnels. Once the ore was at the surface, it was reduced and extracted in smelting ovens. The conditions in Sicilian sulfur mines were horrific, prompting Booker T. Washington to write "I am not prepared just now to say to what extent I believe in a physical hell in the next world, but a sulfur mine in Sicily is about the nearest thing to hell that I expect to see in this life." Sulfur is still mined from surface deposits in poorer nations with volcanoes, such as Indonesia
Indonesia, officially the Republic of Indonesia, is a country in Southeast Asia and Oceania, between the Indian Ocean, Indian and Pacific Ocean, Pacific oceans. Comprising over List of islands of Indonesia, 17,000 islands, including Sumatra, ...
, and problems with working conditions still exist.
Elemental sulfur was extracted from salt domes (where it sometimes occurs in nearly pure form) until the late 20th century, when it became a side product of other industrial processes such as in oil refining, in which sulfur is undesirable. As a mineral, native sulfur under salt domes is thought to be a fossil mineral resource, produced by the action of anaerobic bacteria on sulfate deposits. It was removed from such salt-dome mines mainly by the Frasch process. In this method, superheated water was pumped into a native sulfur deposit to melt the sulfur, and then compressed air returned the 99.5% pure melted product to the surface. Throughout the 20th century this procedure produced elemental sulfur that required no further purification. Due to a limited number of such sulfur deposits and the high cost of working them, this process for mining sulfur has not had significant use anywhere in the world since 2002.
 Since then, sulfur has typically been produced from petroleum, natural gas, and related fossil resources, from which it is obtained mainly as hydrogen sulfide. Organosulfur compounds, undesirable impurities in petroleum, may be upgraded by subjecting them to hydrodesulfurization, which cleaves the C–S bonds:
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by the Claus process, which entails oxidation of some hydrogen sulfide to sulfur dioxide and then the comproportionation of the two:
Since then, sulfur has typically been produced from petroleum, natural gas, and related fossil resources, from which it is obtained mainly as hydrogen sulfide. Organosulfur compounds, undesirable impurities in petroleum, may be upgraded by subjecting them to hydrodesulfurization, which cleaves the C–S bonds:
The resulting hydrogen sulfide from this process, and also as it occurs in natural gas, is converted into elemental sulfur by the Claus process, which entails oxidation of some hydrogen sulfide to sulfur dioxide and then the comproportionation of the two:
Alberta
Alberta is a Provinces and territories of Canada, province in Canada. It is a part of Western Canada and is one of the three Canadian Prairies, prairie provinces. Alberta is bordered by British Columbia to its west, Saskatchewan to its east, t ...
, Canada. Another way of storing sulfur is as a binder for concrete, the resulting product having some desirable properties (see sulfur concrete).
The world production of sulfur in 2011 amounted to 69 million tonnes (Mt), with more than 15 countries contributing more than 1 Mt each. Countries producing more than 5 Mt are China
China, officially the People's Republic of China (PRC), is a country in East Asia. With population of China, a population exceeding 1.4 billion, it is the list of countries by population (United Nations), second-most populous country after ...
(9.6), the United States
The United States of America (USA), also known as the United States (U.S.) or America, is a country primarily located in North America. It is a federal republic of 50 U.S. state, states and a federal capital district, Washington, D.C. The 48 ...
(8.8), Canada
Canada is a country in North America. Its Provinces and territories of Canada, ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, making it the world's List of coun ...
(7.1) and Russia
Russia, or the Russian Federation, is a country spanning Eastern Europe and North Asia. It is the list of countries and dependencies by area, largest country in the world, and extends across Time in Russia, eleven time zones, sharing Borders ...
(7.1). Production has been slowly increasing from 1900 to 2010; the price was unstable in the 1980s and around 2010.
Applications
Sulfuric acid
Elemental sulfur is used mainly as a precursor to other chemicals. Approximately 85% (1989) is converted tosulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
(H2SO4):
 In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
In 2010, the United States produced more sulfuric acid than any other inorganic industrial chemical. The principal use for the acid is the extraction of phosphate ores for the production of fertilizer manufacturing. Other applications of sulfuric acid include oil refining, wastewater processing, and mineral extraction.
Other important sulfur chemistry
Sulfur reacts directly with methane to give carbon disulfide, which is used to manufacture cellophane and rayon. One of the uses of elemental sulfur is in vulcanization of rubber, where polysulfide chains crosslink organic polymers. Large quantities of sulfites are used to bleachpaper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, Textile, rags, poaceae, grasses, Feces#Other uses, herbivore dung, or other vegetable sources in water. Once the water is dra ...
and to preserve dried fruit. Many surfactants and detergents (e.g. sodium lauryl sulfate) are sulfate derivatives. Calcium sulfate, gypsum (CaSO4·2H2O) is mined on the scale of 100 million tonne
The tonne ( or ; symbol: t) is a unit of mass equal to 1,000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton in the United States to distinguish it from the non-metric units of the s ...
s each year for use in Portland cement and fertilizers.
When silver-based photography
Photography is the visual arts, art, application, and practice of creating images by recording light, either electronically by means of an image sensor, or chemically by means of a light-sensitive material such as photographic film. It is empl ...
was widespread, sodium and ammonium thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
were widely used as "fixing agents". Sulfur is a component of gunpowder ("black powder").
Fertilizer
Amino acids synthesized by living organisms such as methionine and cysteine contain organosulfur groups (thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
and thiol respectively). The antioxidant glutathione protecting many living organisms against free radicals and oxidative stress also contains organic sulfur. Some crop
A crop is a plant that can be grown and harvested extensively for profit or subsistence. In other words, a crop is a plant or plant product that is grown for a specific purpose such as food, Fiber, fibre, or fuel.
When plants of the same spe ...
s such as onion and garlic also produce different organosulfur compounds such as ''syn''-propanethial-''S''-oxide responsible of lacrymal irritation (onions), or diallyl disulfide and allicin (garlic). Sulfates, commonly found in soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
s and groundwater
Groundwater is the water present beneath Earth's surface in rock and Pore space in soil, soil pore spaces and in the fractures of stratum, rock formations. About 30 percent of all readily available fresh water in the world is groundwater. A unit ...
s are often a sufficient natural source of sulfur for plants and bacteria. Atmospheric deposition of sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
(SO2) is also a common artificial source ( coal combustion) of sulfur for the soils. Under normal circumstances, in most agricultural soils, sulfur is not a limiting nutrient for plants and microorganism
A microorganism, or microbe, is an organism of microscopic scale, microscopic size, which may exist in its unicellular organism, single-celled form or as a Colony (biology)#Microbial colonies, colony of cells. The possible existence of unseen ...
s (see Liebig's barrel). However, in some circumstances, soils can be depleted in sulfate, e.g. if this later is leached by meteoric water (rain
Rain is a form of precipitation where water drop (liquid), droplets that have condensation, condensed from Water vapor#In Earth's atmosphere, atmospheric water vapor fall under gravity. Rain is a major component of the water cycle and is res ...
) or if the requirements in sulfur for some types of crops are high. This explains that sulfur is increasingly recognized and used as a component of fertilizers. The most important form of sulfur for fertilizer is calcium sulfate, commonly found in nature as the mineral gypsum (CaSO4·2H2O). Elemental sulfur is hydrophobic (not soluble in water) and cannot be used directly by plants. Elemental sulfur (ES) is sometimes mixed with bentonite
Bentonite ( ) is an Absorption (chemistry), absorbent swelling clay consisting mostly of montmorillonite (a type of smectite) which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has a considerably greater swelli ...
to amend depleted soils for crops with high requirement in organo-sulfur. Over time, oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
abiotic processes with atmospheric oxygen and soil bacteria can oxidize and convert elemental sulfur to soluble derivatives, which can then be used by microorganisms and plants. Sulfur improves the efficiency of other essential plant nutrients, particularly nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
and phosphorus. Biologically produced sulfur particles are naturally hydrophilic due to a biopolymer coating and are easier to disperse over the land in a spray of diluted slurry, resulting in a faster uptake by plants.
The plants requirement for sulfur equals or exceeds the requirement for phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
. It is an essential nutrient for plant growth, root nodule formation of legumes, and immunity and defense systems. Sulfur deficiency has become widespread in many countries in Europe. Because atmospheric inputs of sulfur continue to decrease, the deficit in the sulfur input/output is likely to increase unless sulfur fertilizers are used. Atmospheric inputs of sulfur decrease because of actions taken to limit acid rain
Acid rain is rain or any other form of Precipitation (meteorology), precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists b ...
s.
Fungicide and pesticide
 Elemental sulfur is one of the oldest fungicides and
Elemental sulfur is one of the oldest fungicides and pesticide
Pesticides are substances that are used to control pests. They include herbicides, insecticides, nematicides, fungicides, and many others (see table). The most common of these are herbicides, which account for approximately 50% of all p ...
s. "Dusting sulfur", elemental sulfur in powdered form, is a common fungicide for grapes, strawberry, many vegetables and several other crops. It has a good efficacy against a wide range of powdery mildew diseases as well as black spot. In organic production, sulfur is the most important fungicide. It is the only fungicide used in organically farmed apple production against the main disease apple scab under colder conditions. Biosulfur (biologically produced elemental sulfur with hydrophilic characteristics) can also be used for these applications.
Standard-formulation dusting sulfur is applied to crops with a sulfur duster or from a dusting plane. Wettable sulfur is the commercial name for dusting sulfur formulated with additional ingredients to make it water miscible. It has similar applications and is used as a fungicide
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, ...
against mildew and other mold-related problems with plants and soil.
Elemental sulfur powder is used as an " organic" (i.e., "green") insecticide
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, i ...
(actually an acaricide) against ticks and mite
Mites are small arachnids (eight-legged arthropods) of two large orders, the Acariformes and the Parasitiformes, which were historically grouped together in the subclass Acari. However, most recent genetic analyses do not recover the two as eac ...
s. A common method of application is dusting the clothing or limbs with sulfur powder.
A diluted solution of lime sulfur (made by combining calcium hydroxide with elemental sulfur in water) is used as a dip for pets to destroy ringworm (fungus), mange, and other dermatoses and parasites.
Sulfur candles of almost pure sulfur were burned to fumigate structures and wine barrels, but are now considered too toxic for residences.
Pharmaceuticals
Sulfur (specifically octasulfur, S8) is used in pharmaceutical skin preparations for the treatment ofacne
Acne ( ), also known as ''acne vulgaris'', is a long-term Cutaneous condition, skin condition that occurs when Keratinocyte, dead skin cells and Sebum, oil from the skin clog hair follicles. Typical features of the condition include comedo, ...
and other conditions. It acts as a keratolytic agent and also kills bacteria, fungi, scabies mites, and other parasites. Precipitated sulfur and colloidal sulfur are used, in form of lotions, creams, powders, soaps, and bath additives, for the treatment of acne vulgaris, acne rosacea, and seborrhoeic dermatitis.
Many drugs contain sulfur. Early examples include antibacterial sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the Chemical structure, structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this gro ...
, known as ''sulfa drugs''. A more recent example is mucolytic acetylcysteine. Sulfur is a part of many bacterial defense molecules. Most β-lactam antibiotics, including the penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
s, cephalosporins
The cephalosporins (sg. ) are a class of β-lactam antibiotics originally derived from the fungus ''Acremonium'', which was previously known as ''Cephalosporium''.
Together with cephamycins, they constitute a subgroup of β-lactam antibiotic ...
and monobactams contain sulfur.
Batteries
Due to their high energy density and the availability of sulfur, there is ongoing research in creating rechargeable lithium–sulfur batteries. Until now, carbonate electrolytes have caused failures in such batteries after a single cycle. In February 2022, researchers at Drexel University have not only created a prototypical battery that lasted 4000 recharge cycles, but also found the first monoclinic gamma sulfur that remained stable below 95 degrees Celsius.Biological role
Sulfur is an essential component of all living cells. It is the eighth most abundant element in the human body by weight, about equal in abundance topotassium
Potassium is a chemical element; it has Symbol (chemistry), symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to ...
, and slightly greater than sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the peri ...
and chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
. A human body contains about of sulfur. The main dietary source of sulfur for humans is sulfur-containing amino acids, which can be found in plant and animal proteins.
Transferring sulfur between inorganic and biomolecules
In the 1880s, while studying '' Beggiatoa'' (a bacterium living in a sulfur rich environment), Sergei Winogradsky found that it oxidized hydrogen sulfide (H2S) as an energy source, forming intracellular sulfur droplets. Winogradsky referred to this form of metabolism as inorgoxidation (oxidation of inorganic compounds). Another contributor, who continued to study it was Selman Waksman. Primitive bacteria that live around deep ocean volcanic vents oxidize hydrogen sulfide for their nutrition, as discovered by Robert Ballard. Sulfur oxidizers can use as energy sources reduced sulfur compounds, including hydrogen sulfide, elemental sulfur, sulfite,thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, ...
, and various polythionates (e.g., tetrathionate
The tetrathionate anion, , is a sulfur oxyanion derived from the compound tetrathionic acid, H2S4O6. Two of the sulfur atoms present in the ion are in oxidation state 0 and two are in oxidation state +5. Alternatively, the compound can be vi ...
). They depend on enzymes such as sulfur oxygenase and sulfite oxidase to oxidize sulfur to sulfate. Some lithotrophs can even use the energy contained in sulfur compounds to produce sugars, a process known as chemosynthesis. Some bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
and archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
use hydrogen sulfide in place of water as the electron donor in chemosynthesis, a process similar to photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
that produces sugars and uses oxygen as the electron acceptor. Sulfur-based chemosynthesis may be simplifiedly compared with photosynthesis:
There are bacteria combining these two ways of nutrition: green sulfur bacteria and purple sulfur bacteria. Also sulfur-oxidizing bacteria can go into symbiosis with larger organisms, enabling the later to use hydrogen sulfide as food to be oxidized. Example: the giant tube worm.
There are sulfate-reducing bacteria
Sulfate-reducing microorganisms (SRM) or sulfate-reducing prokaryotes (SRP) are a group composed of sulfate-reducing bacteria (SRB) and sulfate-reducing archaea (SRA), both of which can perform anaerobic respiration utilizing sulfate () as termina ...
, that, by contrast, "breathe sulfate" instead of oxygen. They use organic compounds or molecular hydrogen as the energy source. They use sulfur as the electron acceptor, and reduce various oxidized sulfur compounds back into sulfide, often into hydrogen sulfide. They can grow on other partially oxidized sulfur compounds (e.g. thiosulfates, thionates, polysulfides, sulfites).
There are studies pointing that many deposits of native sulfur in places that were the bottom of the ancient oceans have biological origin. These studies indicate that this native sulfur have been obtained through biological activity, but what is responsible for that (sulfur-oxidizing bacteria or sulfate-reducing bacteria) is still unknown for sure.
Sulfur is absorbed by plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s roots from soil as sulfate and transported as a phosphate ester. Sulfate is reduced to sulfide via sulfite before it is incorporated into cysteine and other organosulfur compounds.
While the plants' role in transferring sulfur to animals by food chains is more or less understood, the role of sulfur bacteria is just getting investigated.
Protein and organic metabolites
In all forms of life, most of the sulfur is contained in two proteinogenic amino acids ( cysteine and methionine), thus the element is present in allprotein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s that contain these amino acids. Some of the sulfur is present in certain metabolites—many of which are cofactors—and sulfated polysaccharides of connective tissue ( chondroitin sulfates, heparin).
 The functionality of a given protein is heavily dependent on its structure. Proteins reach this structure through the process of protein folding, which is facilitated by a variety of intra- and inter-molecular bonds. While much of the folding is driven by the formation of hydrogen bonds, covalent bonding of cysteine residues into disulfide bridges imposes constraints that stabilize particular conformations while preventing others from forming. As the bond energy of a covalent disulfide bridge is higher than the energy of a coordinate bond or hydrophobic interaction, greater numbers of disulfide bridges lead to higher energies required for protein denaturation. Disulfide bonds often serve to stabilize protein structures in the more oxidizing conditions of the extracellular environment. Within the
The functionality of a given protein is heavily dependent on its structure. Proteins reach this structure through the process of protein folding, which is facilitated by a variety of intra- and inter-molecular bonds. While much of the folding is driven by the formation of hydrogen bonds, covalent bonding of cysteine residues into disulfide bridges imposes constraints that stabilize particular conformations while preventing others from forming. As the bond energy of a covalent disulfide bridge is higher than the energy of a coordinate bond or hydrophobic interaction, greater numbers of disulfide bridges lead to higher energies required for protein denaturation. Disulfide bonds often serve to stabilize protein structures in the more oxidizing conditions of the extracellular environment. Within the cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
, disulfide bonds may instead be reduced (i.e. in -SH form) to their constituent cysteine residues by thioredoxins.
Many important cellular enzymes use prosthetic groups ending with sulfhydryl (-SH) moieties to handle reactions involving acyl-containing biochemicals: two common examples from basic metabolism are coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric ac ...
and alpha-lipoic acid. Cysteine-related metabolites homocysteine and taurine are other sulfur-containing amino acids that are similar in structure, but not coded by DNA, and are not part of the primary structure of proteins, take part in various locations of mammalian physiology. Two of the 13 classical vitamins, biotin and thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin – an Nutrient#Micronutrients, essential micronutrient for humans and animals. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosp ...
, contain sulfur, and serve as cofactors to several enzymes.
In intracellular chemistry, sulfur operates as a carrier of reducing hydrogen and its electrons for cellular repair of oxidation. Reduced glutathione, a sulfur-containing tripeptide, is a reducing agent through its sulfhydryl (–SH) moiety derived from cysteine.
Methanogenesis, the route to most of the world's methane, is a multistep biochemical transformation of carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
. This conversion requires several organosulfur cofactors. These include coenzyme M, , the immediate precursor to methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
.
Metalloproteins and inorganic cofactors
Metalloproteins—in which the active site is a transition metal ion (or metal-sulfide cluster) often coordinated by sulfur atoms of cysteine residues—are essential components of enzymes involved in electron transfer processes. Examples include plastocyanin (Cu2+) and nitrous oxide reductase (Cu–S). The function of these enzymes is dependent on the fact that the transition metal ion can undergo redox reactions. Other examples include many zinc proteins, as well as iron–sulfur clusters. Most pervasive are the ferrodoxins, which serve as electron shuttles in cells. In bacteria, the important nitrogenase enzymes contain an Fe–Mo–S cluster and is a catalyst that performs the important function of nitrogen fixation, converting atmospheric nitrogen to ammonia that can be used by microorganisms and plants to make proteins, DNA, RNA, alkaloids, and the other organic nitrogen compounds necessary for life. Sulfur is also present in molybdenum cofactor. :
Sulfate
Deficiency
In humans methionine is an essential amino acid; cysteine is conditionally essential and may be synthesized from non-essential serine via sulfur salvaged from methionine. Sulfur deficiency is uncommon due to the ubiquity of cysteine and methionine in food. Isolated sulfite oxidase deficiency is a rare, fatal genetic disease caused by mutations to sulfite oxidase, which is needed to metabolize sulfites to sulfates.Precautions
Toxicity of sulfur compounds
When sulfur burns in air, it producessulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
. In water, this gas produces sulfurous acid and sulfites; sulfites are antioxidants that inhibit growth of aerobic bacteria and a useful food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar ( pickling), salt ( salting), smoke ( smoking) and sugar ( crystallization), have been used f ...
in small amounts. At high concentrations these acids harm the lungs, eyes, or other tissues. In organisms without lungs such as insects, sulfite in high concentration prevents respiration.
Sulfur trioxide (made by catalysis from sulfur dioxide) and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
are similarly highly acidic and corrosive in the presence of water. Concentrated sulfuric acid is a strong dehydrating agent that can strip available water molecules and water components from sugar and organic tissue.
The burning of coal
Coal is a combustible black or brownish-black sedimentary rock, formed as rock strata called coal seams. Coal is mostly carbon with variable amounts of other Chemical element, elements, chiefly hydrogen, sulfur, oxygen, and nitrogen.
Coal i ...
and/or petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
by industry and power plants generates sulfur dioxide (SO2) that reacts with atmospheric water and oxygen to produce sulfurous acid
Sulfuric(IV) acid (United Kingdom spelling: sulphuric(IV) acid), also known as sulfurous (UK: sulphurous) acid and thionic acid, is the chemical compound with the chemical formula, formula .
Raman spectroscopy, Raman spectra of solutions o ...
(H2SO3). These acids are components of acid rain
Acid rain is rain or any other form of Precipitation (meteorology), precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists b ...
, lowering the pH of soil
Soil, also commonly referred to as earth, is a mixture of organic matter, minerals, gases, water, and organisms that together support the life of plants and soil organisms. Some scientific definitions distinguish dirt from ''soil'' by re ...
and freshwater bodies, sometimes resulting in substantial damage to the environment and chemical weathering of statues and structures. Fuel standards increasingly require that fuel producers extract sulfur from fossil fuels to prevent acid rain formation. This extracted and refined sulfur represents a large portion of sulfur production. In coal-fired power plants, flue gases are sometimes purified. More modern power plants that use synthesis gas extract the sulfur before they burn the gas.
Hydrogen sulfide is about one-half as toxic as hydrogen cyanide
Hydrogen cyanide (formerly known as prussic acid) is a chemical compound with the chemical formula, formula HCN and structural formula . It is a highly toxic and flammable liquid that boiling, boils slightly above room temperature, at . HCN is ...
, and intoxicates by the same mechanism (inhibition of the respiratory enzyme cytochrome oxidase), though hydrogen sulfide is less likely to cause sudden poisonings from small inhaled amounts (near its permissible exposure limit (PEL) of 20 ppm) because of its disagreeable odor. However, its presence in ambient air at concentration over 100–150 ppm quickly deadens the sense of smell, and a victim may breathe increasing quantities without noticing until severe symptoms cause death. Dissolved sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families o ...
and hydrosulfide salts are toxic by the same mechanism.
Notes
See also
* Blue lava * Stratospheric sulfur aerosols * Sulfur assimilation * Sulfur isotope biogeochemistry * Ultra-low-sulfur dieselReferences
Further reading
External links
Sulfur
at '' The Periodic Table of Videos'' (University of Nottingham)
Atomic Data for Sulfur
NIST Physical Measurement Laboratory
Sulfur phase diagram
, Introduction to Chemistry for Ages 13–17
* ttps://extoxnet.orst.edu/pips/sulfur.htm Sulfur and its use as a pesticidebr>The Sulphur Institute
{{Authority control Chemical elements Chalcogens Reactive nonmetals Polyatomic nonmetals Agricultural chemicals Anti-acne preparations Dietary minerals Industrial minerals Inorganic polymers Native element minerals Orthorhombic minerals Minerals in space group 70 Pyrotechnic fuels Chemical elements with primitive orthorhombic structure