Spontaneous Fission on:
[Wikipedia]
[Google]
[Amazon]
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy
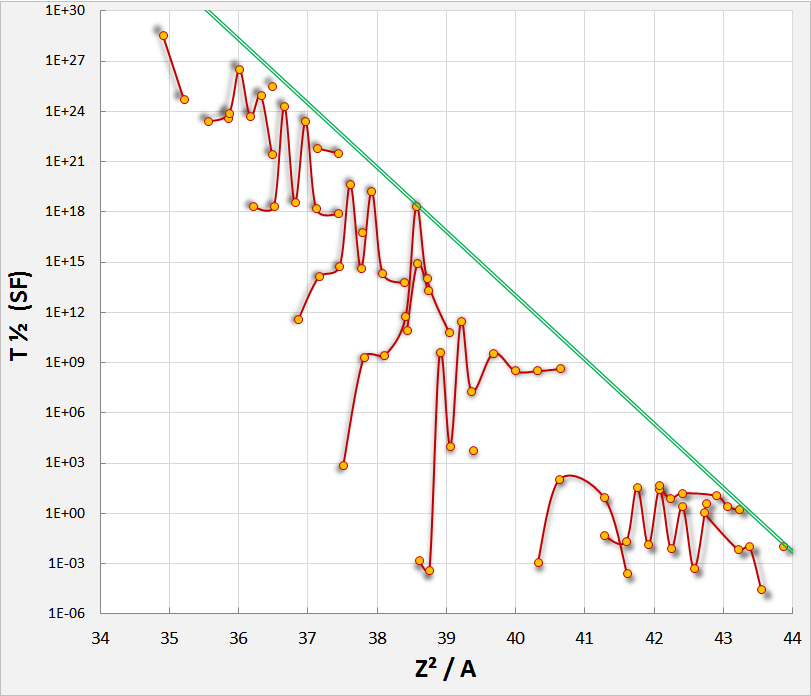 In practice, Pu invariably contains Pu due to the tendency of Pu to absorb an additional neutron during production. Pu's high rate of spontaneous fission makes it an undesirable contaminant. Weapons-grade plutonium contains no more than 7.0% Pu.
The rarely-used gun-type atomic bomb has a critical insertion time of about one millisecond, and the probability of a fission during this time interval should be small. Therefore, only U is suitable. Almost all nuclear bombs use some kind of implosion method.
Spontaneous fission can occur much faster when a nucleus undergoes superdeformation.
In practice, Pu invariably contains Pu due to the tendency of Pu to absorb an additional neutron during production. Pu's high rate of spontaneous fission makes it an undesirable contaminant. Weapons-grade plutonium contains no more than 7.0% Pu.
The rarely-used gun-type atomic bomb has a critical insertion time of about one millisecond, and the probability of a fission during this time interval should be small. Therefore, only U is suitable. Almost all nuclear bombs use some kind of implosion method.
Spontaneous fission can occur much faster when a nucleus undergoes superdeformation.
The LIVEChart of Nuclides - IAEA
'' with filter on spontaneous fission decay {{Nuclear_processes Nuclear physics Nuclear fission Neutron sources Radioactivity
chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
s. The nuclear binding energy of the elements reaches its maximum at an atomic mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxi ...
of about 56 (e.g., iron-56
Iron-56 (56Fe) is the most common isotope of iron. About 91.754% of all iron is iron-56.
Of all nuclides, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei.
...
); spontaneous breakdown into smaller nuclei and a few isolated nuclear particle
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons ...
s becomes possible at greater atomic mass numbers.
History
By 1908, physicists understood thatalpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
involved ejection of helium nuclei from a decaying atom. Like cluster decay, alpha decay is not typically categorized as a process of fission.
The first nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
process discovered was fission induced by neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s. Because cosmic ray
Cosmic rays are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own ...
s produce some neutrons, it was difficult to distinguish between induced and spontaneous events. Cosmic rays can be reliably shielded by a thick layer of rock or water. Spontaneous fission was identified in 1940 by Soviet
The Soviet Union,. officially the Union of Soviet Socialist Republics. (USSR),. was a List of former transcontinental countries#Since 1700, transcontinental country that spanned much of Eurasia from 1922 to 1991. A flagship communist state, ...
physicists Georgy Flyorov
Georgii Nikolayevich Flyorov (also spelled Flerov, rus, Гео́ргий Никола́евич Флёров, p=gʲɪˈorgʲɪj nʲɪkɐˈlajɪvʲɪtɕ ˈflʲɵrəf; 2 March 1913 – 19 November 1990) was a Soviet physicist who is known for h ...
and Konstantin Petrzhak by their observations of uranium in the Moscow Metro Dinamo station, underground.
Feasibility
Elemental
Spontaneous fission occurs over practical observation times only for atomic masses of 232 atomic mass units or more. These are nuclei at least as heavy as thorium-232which has ahalf-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
somewhat longer than the age of the universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the univers ...
. Th, U, and U are primordial nuclide
In geochemistry, geophysics and nuclear physics, primordial nuclides, also known as primordial isotopes, are nuclides found on Earth that have existed in their current form since before Earth was formed. Primordial nuclides were present in the ...
s and have left evidence of undergoing spontaneous fission in their minerals.
The known elements most susceptible to spontaneous fission are the synthetic high-atomic-number actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s and transactinide
Superheavy elements, also known as transactinide elements, transactinides, or super-heavy elements, are the chemical elements with atomic number greater than 103. The superheavy elements are those beyond the actinides in the periodic table; the l ...
s with atomic number 100 onward.
For naturally occurring thorium-232, uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exi ...
, and uranium-238, spontaneous fission does occur rarely, but in the vast majority of the radioactive decay of these atoms, alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
or beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
occurs instead. Hence, the spontaneous fission of these isotopes is usually negligible, except in using the exact branching ratios when finding the radioactivity of a sample of these elements, or in applications that are very sensitive to even minuscule numbers of fission neutrons (such as nuclear weapon design).
Mathematical
The liquid drop model predicts approximately that spontaneous fission can occur in a time short enough to be observed by present methods when : where ''Z'' is theatomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
and ''A'' is the mass number (e.g., for uranium-235). However, no known radioactive isotope except oganesson-294 reaches a value of 47 (approximately 47.36), as the liquid drop model is not very accurate for the heaviest known nuclei due to strong shell effects.
Spontaneous fission rates
 In practice, Pu invariably contains Pu due to the tendency of Pu to absorb an additional neutron during production. Pu's high rate of spontaneous fission makes it an undesirable contaminant. Weapons-grade plutonium contains no more than 7.0% Pu.
The rarely-used gun-type atomic bomb has a critical insertion time of about one millisecond, and the probability of a fission during this time interval should be small. Therefore, only U is suitable. Almost all nuclear bombs use some kind of implosion method.
Spontaneous fission can occur much faster when a nucleus undergoes superdeformation.
In practice, Pu invariably contains Pu due to the tendency of Pu to absorb an additional neutron during production. Pu's high rate of spontaneous fission makes it an undesirable contaminant. Weapons-grade plutonium contains no more than 7.0% Pu.
The rarely-used gun-type atomic bomb has a critical insertion time of about one millisecond, and the probability of a fission during this time interval should be small. Therefore, only U is suitable. Almost all nuclear bombs use some kind of implosion method.
Spontaneous fission can occur much faster when a nucleus undergoes superdeformation.
Poisson process
Spontaneous fission gives much the same result as inducednuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
. However, like other forms of radioactive decay, it occurs due to quantum tunneling, without the atom having been struck by a neutron or other particle as in induced nuclear fission. Spontaneous fissions release neutrons as all fissions do, so if a critical mass is present, a spontaneous fission can initiate a self-sustaining chain reaction. Radioisotopes for which spontaneous fission is not negligible can be used as neutron sources. For example, californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding c ...
-252 (half-life 2.645 years; SF branch ratio 3.1%) can be used for this purpose. The neutrons released can be used to inspect airline luggage for hidden explosives, to gauge the moisture content of soil in highway
A highway is any public or private road or other public way on land. It is used for major roads, but also includes other public roads and public tracks. In some areas of the United States, it is used as an equivalent term to controlled-access ...
and building construction, or to measure the moisture of materials stored in silos, for example.
As long as the spontaneous fission gives a negligible reduction of the number of nuclei that can undergo such fission, this process can be approximated closely as a Poisson process. In this situation, for short time intervals the probability of a fission is directly proportional to the length of time.
The spontaneous fission of uranium-238 and uranium-235 leaves trails of damage in the crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
of uranium-containing minerals when the fission fragments recoil through them. These trails, or ''fission tracks'', are the foundation of the radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares ...
method called fission track dating.
See also
*Natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. ...
Notes
External links
* 'The LIVEChart of Nuclides - IAEA
'' with filter on spontaneous fission decay {{Nuclear_processes Nuclear physics Nuclear fission Neutron sources Radioactivity