Stieglitz Rearrangement on:
[Wikipedia]
[Google]
[Amazon]
The Stieglitz rearrangement is a
 The Stieglitz rearrangement's reaction mechanism and the products and starting materials involved make it closely related to the
The Stieglitz rearrangement's reaction mechanism and the products and starting materials involved make it closely related to the 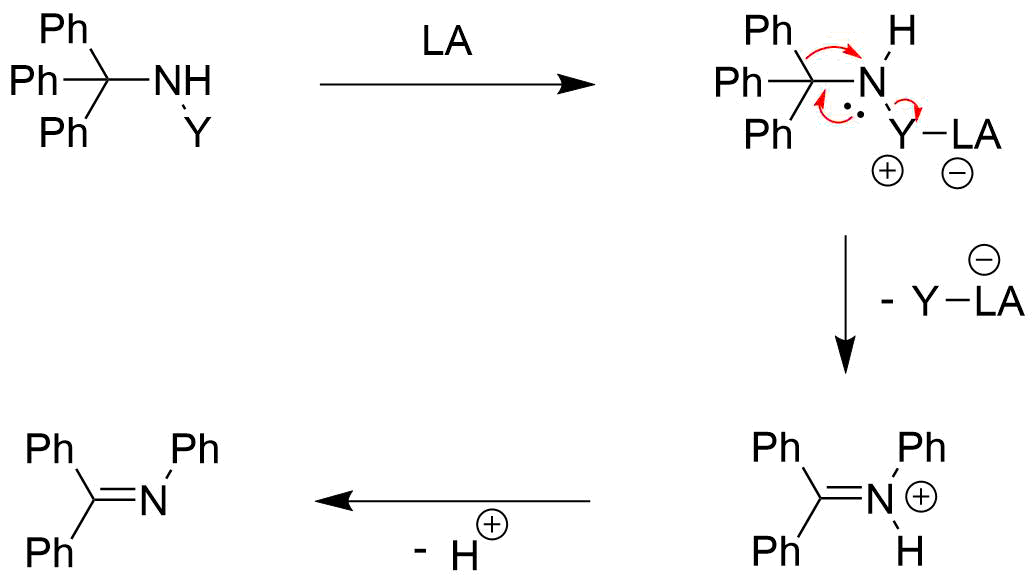

 However, there also have been reported examples of base-free Stieglitz rearrangements of ''N''-halogenated amines. An example for that can be found in the total synthesis of (±)-lycopodine by Paul Grieco ''et al.'' There, a ring formation takes place by a rearrangement on a secondary haloamine by subjecting it to silver tetrafluoroborate. AgBF4 is known to act as a source of Ag+ ions that can facilitate the dissociation of halides from organic molecules, with the formation of the respective silver halide as a driving force. The desired product is then obtained by reduction with
However, there also have been reported examples of base-free Stieglitz rearrangements of ''N''-halogenated amines. An example for that can be found in the total synthesis of (±)-lycopodine by Paul Grieco ''et al.'' There, a ring formation takes place by a rearrangement on a secondary haloamine by subjecting it to silver tetrafluoroborate. AgBF4 is known to act as a source of Ag+ ions that can facilitate the dissociation of halides from organic molecules, with the formation of the respective silver halide as a driving force. The desired product is then obtained by reduction with 
rearrangement reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another at ...
in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
which is named after the American chemist Julius Stieglitz
Julius Oscar Stieglitz (May 26, 1867 – January 10, 1937) was an American chemist of German Jewish origin. He was a teacher and organic chemist with a major interest in pharmaceutical and medicinal chemistry. He is known for the Stieglitz rear ...
(1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Frank C. Whitmore, Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atom ...
of trityl
Triphenylmethane or triphenyl methane (sometimes also known as Tritan), is the hydrocarbon with the chemical formula, formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic sk ...
amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
derivatives to triaryl imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s. It is comparable to a Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement reaction, rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on ...
which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
after activation with phosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula . It is one of the most important phosphorus chlorides/oxychlorides, others being and . finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, althoug ...
to yield the respective triaryl imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
, a Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldim ...
.
In general, the term "Stieglitz rearrangement" is used to describe a wide variety of rearrangement reactions of amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
s to imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
s. Although, it is generally associated with the rearrangement of triaryl hydroxylamine
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Prof ...
s, that are well-reported in the academic literature, Stieglitz rearrangements can also occur on alkylated Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
derivatives, haloamines and azide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
s as well as other activated amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
derivatives.
General mechanism and relatedness to the Beckmann rearrangement
Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement reaction, rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on ...
, which can be used for the synthesis of carboxamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a pe ...
s. Both rearrangement reactions involve a carbon to nitrogen shift, usually after electrophilic activation of the leaving group on the nitrogen atom. The main difference in the starting materials, however, is their saturation degree. While a Stieglitz rearrangement takes place on saturated amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
derivatives with a σ-single bond, the typical starting material for a Beckmann rearrangement is an oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
(a hydroxylimine) with a C=N-double bond.
In a Beckmann rearrangement, the acid catalyzed carbon to nitrogen migration takes place on the oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
to yield a nitrilium ion intermediate. In principle, the first step of a Stieglitz rearrangement proceeds in an analogous way. However, after the generation of the positively charged iminium ion through the π-interaction between the nitrogen lone pair and the electron deficient carbon in the Stieglitz rearrangement, the pathways diverge. In the Stieglitz rearrangement, a charge-neutral state of the molecule can be achieved by dissociation of a proton. Alternatively, if the starting material did not possess any amino protons, the neutral state can be achieved with an external reducing agent, such as sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
. It reduces the iminium ion intermediate to the corresponding saturated amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
. In the Beckmann rearrangement such a proton is also missing and the stabilization of the intermediate proceeds via a nucleophilic addition of a water molecule, dissociation of a proton and tautomerism from the imidic acid
In organic chemistry, imidic acids are organic compounds with the structure . They are tautomeric to non- tertiary amides () and isomeric to oximes ().
The term " imino acid" is an obsolete term for this group that should not be used in this c ...
to the carboxamide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a pe ...
.

Variations
Although the original Stieglitz reaction is best known for the rearrangement of trityl hydroxylamines, there are several variations which include good leaving groups as ''N''-substituents (such as halogens and sulfonates). Different reagents are commonly applied, depending on the exact nature of the substrate.Stieglitz rearrangement of ''N''-hydroxylated amines, ''N''-alkoxylated amines and ''N''-sulfonated amines
Stieglitz rearrangement of ''N''-hydroxylated amines
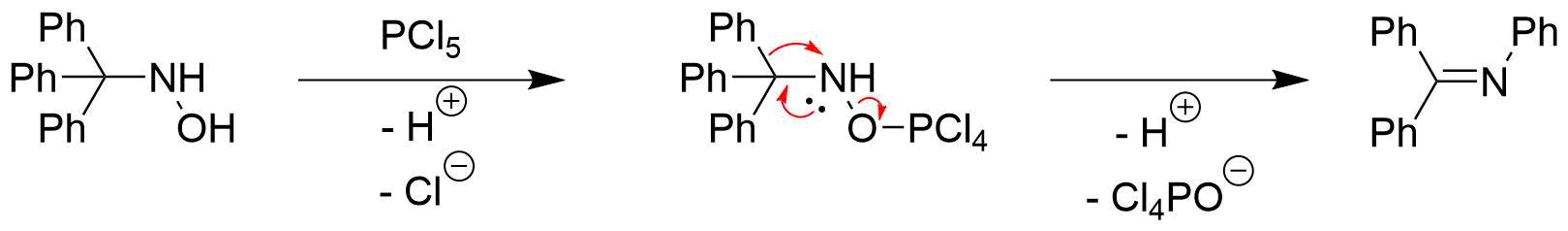
For the rearrangement of trityl hydroxylamines, Lewis acids such as phosphorus pentachloride (PCl5), phosphorus pentoxide (P2O5) or boron trifluoride (BF3) can be used. They function as electrophilic activators for thehydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
by increasing the quality of the leaving group.
For example, when using PCl5 as a reagent, the trityl hydroxylamine is first transformed into the activated intermediate via a nucleophilic substitution. The generated intermediate can then undergo rearrangement by the migration of the phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
and dissociation of the phosphorus(V) species to form ''N''-phenyl benzophenone imine.

Stieglitz rearrangement of ''N''-alkoxylated amines
Additionally to ''N''-hydroxy trityl amines, rearrangements in ''N''-alkoxy trityl amines are also possible. However, those reactions are known for their intrinsically low yields. For example, ''N''-benzyloxy substituted trityl amine can undergo a Stieglitz rearrangement in the presence of phosphorus pentachloride (160 °C, 40% yield) or with BF3 as a reagent (60 °C, 29% yield). In the latter case, BF3 acts as a Lewis acid in the electrophilic activation of the benzylic oxygen to allow for a nucleophilic attack on the adjacent nitrogen atom.
Stieglitz rearrangement of ''N''-sulfonated amines
Stieglitz rearrangements also readily proceed with active sulfonates as a leaving group. ''N''-sulfonated amines can be obtained from the respective hydroxylamines and suitable sulfonation reagents. For example, Herderin ''et al.'' synthesized their secondary hydroxylamine (starting material in the rearrangement shown below) by subjecting the respective hydroxylamine totosyl chloride
4-Toluenesulfonyl chloride (''p''-toluenesulfonyl chloride, toluene-''p''-sulfonyl chloride) is an organic compound with the formula CH3C6H4SO2Cl. This white, malodorous solid is a reagent widely used in organic synthesis. Abbreviated TsCl or To ...
and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula . It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly corrosive base (chemistry), ...
in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
.
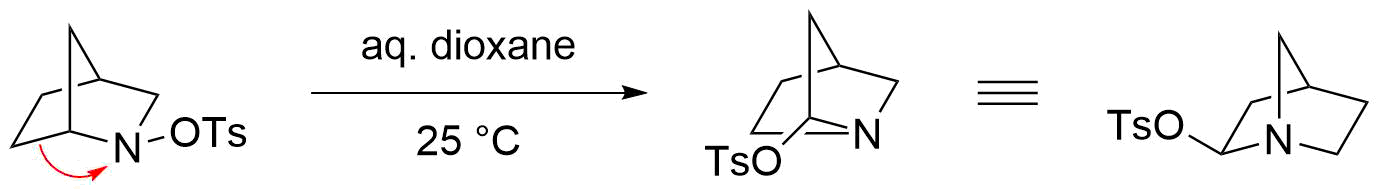
The Stieglitz rearrangement is especially reactive in the case of bridged bicyclic ''N''-sulfonated amines as starting materials, where mild conditions are sufficient for an efficient reaction to take place. For example, the rearrangement of the bicyclic ''N''-tosylated amine proceeds readily in aqueous dioxane at room temperature. However, the respective imine is not formed in this case, presumably due to the strain that would thermodynamically disfavor such a structure, bearing a double bond at a bridgehead atom (Bredt's rule
In organic chemistry, an anti-Bredt molecule is a Bridged compound, bridged molecule with a double bond at the Bicyclic molecule, bridgehead. Bredt's rule is the empirical observation that such molecules only form in large ring systems. For exam ...
). Instead, the tosylate is nucleophilically added at the geminal position of the nitrogen via an attack on the iminium ion.

Stieglitz rearrangement of azides
Stieglitz rearrangements can also proceed on organicazide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
s with molecular nitrogen as a good leaving group. Those reactions proceed comparably to steps of the Schmidt reaction
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. ...
, by which carboxylic acids can be transformed into amines through the addition of hydrazoic acid
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, This also contains a detailed description of the contemporaneous production process. is a compound with the chemical formula . It is a colorless, volatile, and explosive liquid ...
under acidic aqueous conditions.
The Stieglitz rearrangement of azides generally profits from a protonic or thermal activation, which can also be combined. In both cases, molecular nitrogen is set free as a gas in an irreversible step. It has been suggested that the rearrangement, after the dissociation of the N2 molecule, proceeds over a reactive nitrene
In chemistry, a nitrene or imene () is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and valence (chemistry)#monovalent, monovalent, so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded e ...
intermediate. These intermediates would be quite similar to those that have been proposed to be key intermediates in the rearrangement reactions named after Hofmann and Curtius, but have since been considered unlikely.
When subjecting the azide to a Brønsted acid, the protonation of the azide activates the basal nitrogen and lowers the bond strength to the adjacent one, so that the dissociation and expulsion of molecular nitrogen is eased. After the rearrangement the proton can then dissociate from the iminium ion to yield the imine.
An alternative way for the production of protonated organic azides is the nuclophilic addition of hydrazoic acid to a carbocations, which can then also undergo Stieglitz rearrangements.
Stieglitz rearrangement of ''N''-halogenated amines
The Stieglitz rearrangement of ''N''-halogenated amines can be observed for chlorine and bromine substituted amines, often in combination with an organic base, such assodium methoxide
Sodium methoxide is the simplest sodium alkoxide. With the formula , it is a white solid, which is formed by the deprotonation of methanol. It is a widely used reagent in industry and the laboratory. It is also a dangerously caustic base.
...
. The need for a base is generally affiliated with the need for a deprotonation of the amine.
 However, there also have been reported examples of base-free Stieglitz rearrangements of ''N''-halogenated amines. An example for that can be found in the total synthesis of (±)-lycopodine by Paul Grieco ''et al.'' There, a ring formation takes place by a rearrangement on a secondary haloamine by subjecting it to silver tetrafluoroborate. AgBF4 is known to act as a source of Ag+ ions that can facilitate the dissociation of halides from organic molecules, with the formation of the respective silver halide as a driving force. The desired product is then obtained by reduction with
However, there also have been reported examples of base-free Stieglitz rearrangements of ''N''-halogenated amines. An example for that can be found in the total synthesis of (±)-lycopodine by Paul Grieco ''et al.'' There, a ring formation takes place by a rearrangement on a secondary haloamine by subjecting it to silver tetrafluoroborate. AgBF4 is known to act as a source of Ag+ ions that can facilitate the dissociation of halides from organic molecules, with the formation of the respective silver halide as a driving force. The desired product is then obtained by reduction with sodium cyanoborohydride
Sodium cyanoborohydride is a chemical compound with the formula . It is a colourless salt used in organic synthesis for chemical reduction including that of imines and carbonyls. Sodium cyanoborohydride is a milder reductant than other convention ...
, a mild reducing agent which is commonly employed in the reduction of imines to amines.

Stieglitz rearrangement of lead tetraacetate-activated amines
It was also observed, that the addition oflead tetraacetate
Lead(IV) acetate or lead tetraacetate is an metalorganic compound with chemical formula , often abbreviated as , where Ac is acyl. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is de ...
can facilitate the Stieglitz rearrangement of amine derivatives. After the formation of the activated amine derivative intermediate by coordination to the lead center, the following rearrangement again proceeds via migration of the aromatic group under formation of a C–N bond, dissociation of lead and the deprotonation of the resulting iminium ion.
See also
*Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement reaction, rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on ...
* Curtius rearrangement
The Curtius rearrangement (or Curtius reaction or Curtius degradation), first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a ...
* Dakin oxidation
The Dakin oxidation (or Dakin reaction) is an organic chemistry, organic redox, redox reaction in which an ''arene substitution patterns, ortho''- or ''arene substitution patterns, para''-hydroxylated phenyl group, phenyl aldehyde (2-hydroxybe ...
* Schmidt reaction
In organic chemistry, the Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl derivative, usually an aldehyde, ketone, or carboxylic acid, under acidic conditions to give an amine or amide, with expulsion of nitrogen. ...
References
{{Reflist Rearrangement reactions Name reactions