Steam distillation on:
[Wikipedia]
[Google]
[Amazon]
 Steam distillation is a
Steam distillation is a
Steam distillation applied to the food industry
. Chapter 2 of ''Extracting Bioactive Compounds for Food Products: Theory and Applications'', pages 9–75.
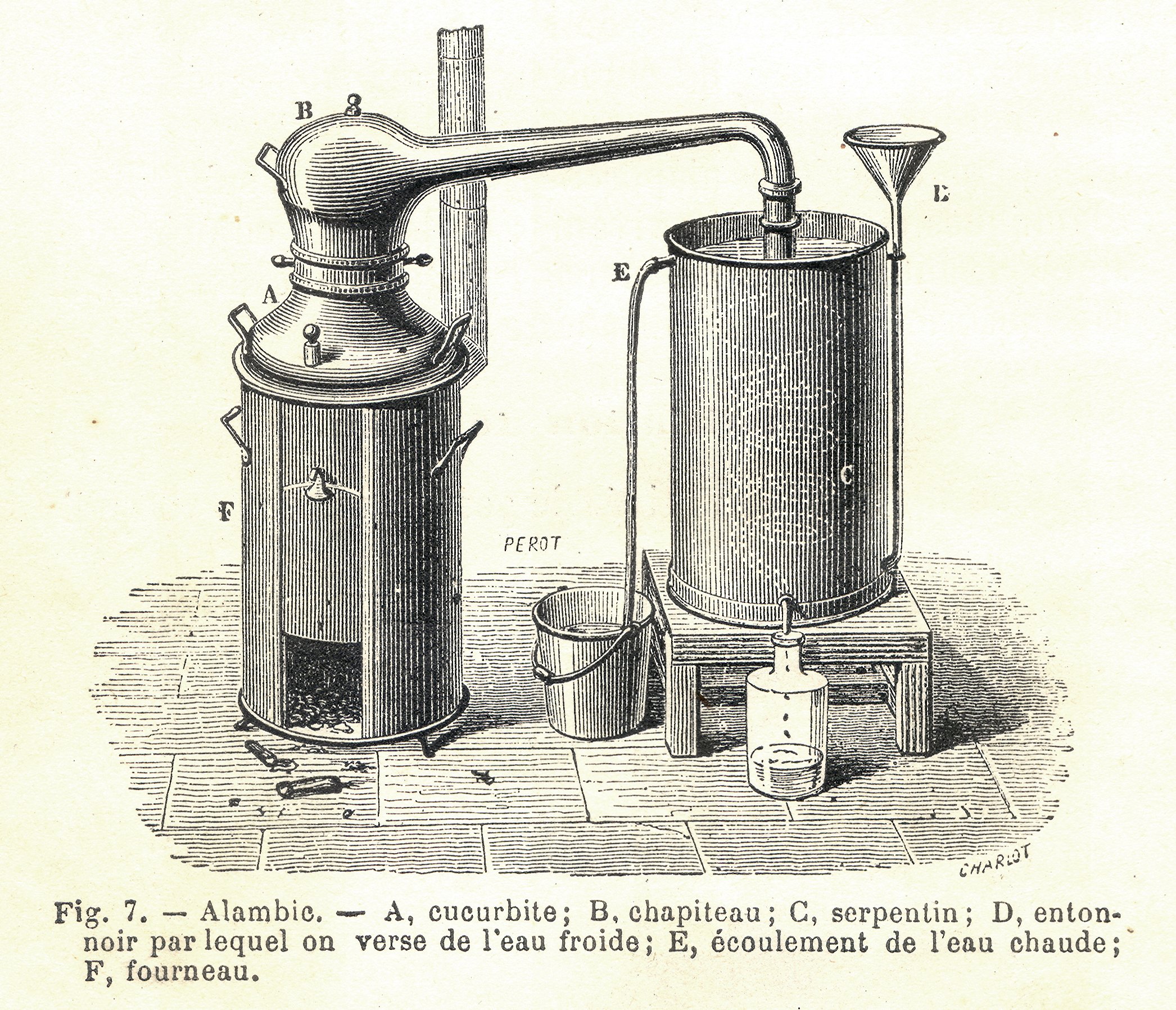 Steam distillation is used in many of the recipes given in the ('Book of Gentleness on Perfume'), also known as the ('Book of the Chemistry of Perfume and Distillations'), attributed to the early Arabic philosopher
Steam distillation is used in many of the recipes given in the ('Book of Gentleness on Perfume'), also known as the ('Book of the Chemistry of Perfume and Distillations'), attributed to the early Arabic philosopher
 Steam distillation is often employed in the isolation of
Steam distillation is often employed in the isolation of
 Steam distillation is a
Steam distillation is a separation process
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substances in order to obtain purity. At least one product mi ...
that consists of distilling
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixt ...
water together with other volatile and non-volatile components. The steam
Steam is water vapor, often mixed with air or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Saturated or superheated steam is inv ...
from the boiling water carries the vapor of the volatiles to a condenser; both are cooled and return to the liquid or solid state, while the non-volatile residues remain behind in the boiling container.
If, as is usually the case, the volatiles are not miscible with water, they will spontaneously form a distinct phase after condensation, allowing them to be separated by decantation
Decantation is a process for the separation of mixtures of miscible, immiscible liquids or of a liquid and a solid mixture such as a Suspension (chemistry), suspension. The layer closer to the top of the container—the less density, dense of th ...
or with a separatory funnel
A separatory funnel, also known as a separation funnel, separating funnel, or colloquially sep funnel, is a piece of laboratory glassware used in liquid-liquid extractions to separate (''partition'') the components of a mixture into two miscible, i ...
.
Steam distillation can be used when the boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of the substance to be extracted is higher than that of water, and the starting material cannot be heated to that temperature because of decomposition
Decomposition is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ess ...
or other unwanted reactions. It may also be useful when the amount of the desired substance is small compared to that of the non-volatile residues. It is often used to separate volatile essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the ...
s from plant material. for example, to extract limonene
Limonene () is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the essential oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, ...
(boiling point 176 °C) from orange peels.
Steam distillation once was a popular laboratory method for purification of organic compounds, but it has been replaced in many such uses by vacuum distillation and supercritical fluid extraction. It is however much simpler and economical than those alternatives, and remains important in certain industrial sectors.Zeki Berk (2018): ''Food Process Engineering and Technology'', 3rd edition. 742 pages.
In the simplest form, water distillation or hydrodistillation, the water is mixed with the starting material in the boiling container. In direct steam distillation, the starting material is suspended above the water in the boiling flask, supported by a metal mesh
Medical Subject Headings (MeSH) is a comprehensive controlled vocabulary for the purpose of indexing journal articles and books in the life sciences. It serves as a thesaurus of index terms that facilitates searching. Created and updated by th ...
or perforated screen. In dry steam distillation, the steam from a boiler
A boiler is a closed vessel in which fluid (generally water) is heated. The fluid does not necessarily boil. The heated or vaporized fluid exits the boiler for use in various processes or heating applications, including water heating, centra ...
is forced to flow through the starting material in a separate container. The latter variant allows the steam to be heated above the boiling point of water (thus becoming superheated steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured.
Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of its ...
), for more efficient extraction.Manuel G. Cerpa, Rafael B. Mato, María José Cocero, Roberta Ceriani, Antonio J. A. Meirelle, Juliana M. Prado, Patrícia F. Leal, Thais M. Takeuchi, and M. Angela A. Meireles (2008):Steam distillation applied to the food industry
. Chapter 2 of ''Extracting Bioactive Compounds for Food Products: Theory and Applications'', pages 9–75.
History
 Steam distillation is used in many of the recipes given in the ('Book of Gentleness on Perfume'), also known as the ('Book of the Chemistry of Perfume and Distillations'), attributed to the early Arabic philosopher
Steam distillation is used in many of the recipes given in the ('Book of Gentleness on Perfume'), also known as the ('Book of the Chemistry of Perfume and Distillations'), attributed to the early Arabic philosopher al-Kindi
Abū Yūsuf Yaʻqūb ibn ʼIsḥāq aṣ-Ṣabbāḥ al-Kindī (; ; ; ) was an Arab Muslim polymath active as a philosopher, mathematician, physician, and music theorist
Music theory is the study of theoretical frameworks for understandin ...
(–873). Steam distillation was also used by the Persian philosopher and physician Avicenna
Ibn Sina ( – 22 June 1037), commonly known in the West as Avicenna ( ), was a preeminent philosopher and physician of the Muslim world, flourishing during the Islamic Golden Age, serving in the courts of various Iranian peoples, Iranian ...
(980–1037) to produce essential oils by adding water to rose petals and distilling the mixture. The process was also used by al-Dimashqi (1256–1327) to produce rose water
Rose water, or rosewater, is a flavoured water created by steeping rose petals in water. It is typically made as a by-product during the distillation of rose petals to create rose oil for perfumes. Rose water is widely utilized to flavour cu ...
on a large scale.
Principle
Every substance has somevapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indicat ...
even below its boiling point, so in theory it could be distilled at any temperature by collecting and condensing its vapors. However, ordinary distillation below the boiling point is not practical because a layer of vapor-rich air would form over the liquid, and evaporation would stop as soon as the partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
of the vapor in that layer reached the vapor pressure. The vapor would then flow to the condenser only by diffusion, which is an extremely slow process.
Simple distillation is generally done by boiling the starting material, because, once its vapor pressure exceeds atmospheric pressure, that still vapor-rich layer of air will be disrupted, and there will be a significant and steady flow of vapor from the boiling flask to the condenser.
In steam distillation, that positive flow is provided by steam from boiling water, rather than by the boiling of the substances of interest. The steam carries with it the vapors of the latter.
The substance of interest does not need to be miscible water or soluble in it. It suffices that it has significant vapor pressure at the steam's temperature.
If the water forms an azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens beca ...
with the substances of interest, the boiling point of the mixture may be lower than the boiling point of water. For example, bromobenzene boils at 156 °C (at normal atmospheric pressure), but a mixture with water boils at 95 °C. However, the formation of an azeotrope is not necessary for steam distillation to work.
Applications
essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the ...
s, for use in perfumes
Perfume (, ) is a mixture of fragrance, fragrant essential oils or aroma compounds (fragrances), Fixative (perfumery), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agre ...
, for example. In this method, steam is passed through the plant material containing the desired oils. Eucalyptus oil
Eucalyptus oil is the generic name for distilled oil from the leaves of ''Eucalyptus'', a genus of the plant family Myrtaceae, mostly native to Australia but cultivated worldwide. Eucalyptus oil has a history of wide application, as a pharmace ...
, camphor oil and orange oil are obtained by this method on an industrial scale. Steam distillation is a means of purifying fatty acids, e.g. from tall oils.
Steam distillation is sometimes used in the chemical laboratory. Illustrative is a classic preparation of bromobiphenyl
Biphenyl (also known as diphenyl, phenylbenzene, 1,1′-biphenyl, lemonene or BP) is an organic compound that forms colorless crystals. Particularly in older literature, compounds containing the functional group consisting of biphenyl less one ...
where steam distillation is used to first remove the excess benzene and subsequently to purifiy the brominated product. In one preparation of benzophenone
Benzophenone is a naturally occurring organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone has been found in some fungi, fruits and plants, including grapes. It is a white solid with a low melting point and ros ...
, steam is employed to first recover unreacted carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a n ...
and subsequently to hydrolyze the intermediate benzophenone dichloride into benzophenone, which is in fact not steam distilled. It one preparation of a purine
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted puri ...
, steam distillation is used to remove volatile benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-li ...
from nonvolatile product.
Equipment
On a lab scale, steam distillations are carried out using steam generated outside the system and piped through the mixture to be purified. Steam can also be generated in-situ using a Clevenger-type apparatus. The Likens-Nickerson apparatus simultaneously performs steam distillation and extraction. It is typically used to isolate targetorganic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s for further analysis.
See also
*Azeotropic distillation
In chemistry, azeotropic distillation is any of a range of techniques used to break an azeotrope in distillation. In chemical engineering, ''azeotropic distillation'' usually refers to the specific technique of adding another component to genera ...
* Batch distillation
* Distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixt ...
* Extractive distillation
* Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
* Heteroazeotrope
* Herbal distillates
Herbal distillates, also known as floral waters, hydrosols, hydrolates, herbal waters, and essential waters, are aqueous products of hydrodistillation. They are colloidal suspensions of essential oils as well as water-soluble components obtained ...
* Hydrodistillation
* Laboratory equipment
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratories are found in a variety of settings such as schools, u ...
* Steam engine
A steam engine is a heat engine that performs Work (physics), mechanical work using steam as its working fluid. The steam engine uses the force produced by steam pressure to push a piston back and forth inside a Cylinder (locomotive), cyl ...
* Steam stripping
* Supercritical fluid extraction
* Theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as ...
References
{{DEFAULTSORT:Steam Distillation Distillation