silicone rubber on:
[Wikipedia]
[Google]
[Amazon]
 Silicone rubber is an
Silicone rubber is an
 Two-part condensation systems package the cross-linker and condensation catalyst together in one part while the polymer and any fillers or pigments are in the second part. Mixing of the two parts causes the curing to take place. A typical filler is fumed silica, also known as pyrogenic silica, which used to control the flow properties of the sealant.
Once fully cured, condensation systems are effective as sealants and caulks in plumbing and building construction and as molds for casting polyurethane, epoxy, and polyester resins, waxes, gypsum, and low-melting-temperature metals such as lead. They are typically very flexible and have a high tear strength. They do not require the use of a release agent since silicones have non-stick properties.
Two-part condensation systems package the cross-linker and condensation catalyst together in one part while the polymer and any fillers or pigments are in the second part. Mixing of the two parts causes the curing to take place. A typical filler is fumed silica, also known as pyrogenic silica, which used to control the flow properties of the sealant.
Once fully cured, condensation systems are effective as sealants and caulks in plumbing and building construction and as molds for casting polyurethane, epoxy, and polyester resins, waxes, gypsum, and low-melting-temperature metals such as lead. They are typically very flexible and have a high tear strength. They do not require the use of a release agent since silicones have non-stick properties.


 Polysiloxanes differ from other polymers in that their backbones consist of Si–O–Si units instead of C–C units. The large bond angles and bond lengths make polysiloxanes more flexible than basic polymers such as
Polysiloxanes differ from other polymers in that their backbones consist of Si–O–Si units instead of C–C units. The large bond angles and bond lengths make polysiloxanes more flexible than basic polymers such as
 Silicone rubber can be moulded into custom shapes and designs. Manufacturers work to set industry tolerances when extruding, cutting, or joining silicone rubber profiles. In the UK this is BS 3734, for extrusions the tightest level is E1 and the widest is E3.
Silicone rubber can be moulded into custom shapes and designs. Manufacturers work to set industry tolerances when extruding, cutting, or joining silicone rubber profiles. In the UK this is BS 3734, for extrusions the tightest level is E1 and the widest is E3.
 Silicone rubber can be 3d printed (liquid deposition modelling LDM) using pump-nozzle extrusion systems. Unfortunately, standard silicone formulations are optimized to be used by extrusion and injection moulding machines and are not applicable in LDM-based 3D printing. The rheological behavior and the
Silicone rubber can be 3d printed (liquid deposition modelling LDM) using pump-nozzle extrusion systems. Unfortunately, standard silicone formulations are optimized to be used by extrusion and injection moulding machines and are not applicable in LDM-based 3D printing. The rheological behavior and the
elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''ela ...
composed of silicone
In Organosilicon chemistry, organosilicon and polymer chemistry, a silicone or polysiloxane is a polymer composed of repeating units of siloxane (, where R = Organyl group, organic group). They are typically colorless oils or elastomer, rubber ...
—itself a polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
—containing silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
together with carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
, and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost.
Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including voltage line insulators; automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware, in products such as silicone sealants.
The term "silicone" is actually a misnomer. The suffix ''-one'' is used by chemists to denote a substance with a double-bonded atom of oxygen in its backbone. When first discovered, silicone was erroneously believed to have oxygen atoms bonded in this way. The technically correct term for the various silicone rubbers is polysiloxanes (polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication and passive daytime radiative cooling.
PDMS is particularly known for its ...
s being a large subset), referring to a saturated Si-O backbone.
Curing
In its uncured state, silicone rubber is a highly adhesive gel or liquid. To convert it to a solid, it must be cured, vulcanized, or catalyzed. This is normally carried out in a two-stage process at the point of manufacture into the desired shape, and then in a prolonged post-cure process. It can also beinjection molded
Injection or injected may refer to:
Science and technology
* Injective function, a mathematical function mapping distinct arguments to distinct values
* Injection (medicine), insertion of liquid into the body with a syringe
* Injection, in broadca ...
or 3D printed.
Silicone rubber may be cured by a platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
-catalyzed cure system, a condensation cure system, a peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
cure system, or an oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
cure system. For the platinum-catalyzed cure system, the curing process can be accelerated by adding heat or pressure.
Platinum-based cure system
In a platinum-based silicone cure system, also called an ''addition'' system (because the key reaction-building polymer is anaddition reaction
In organic chemistry, an addition reaction is an organic reaction in which two or more molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, ...
), a hydride
In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen ion with two electrons. In modern usage, this is typically only used for ionic bonds, but it is sometimes (and has been more frequently in the past) applied to all che ...
- and a vinyl
Vinyl may refer to:
Chemistry
* Polyvinyl chloride (PVC), a particular vinyl polymer
* Vinyl cation, a type of carbocation
* Vinyl group, a broad class of organic molecules in chemistry
* Vinyl polymer, a group of polymers derived from vinyl ...
-functional siloxane
In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: . The parent siloxanes include the oligomeric and polymeric hydrides with the formulae and . Siloxanes ...
polymer react in the presence of a platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
catalyst, creating an ethyl bridge between the two. The reaction has no byproducts. Such silicone rubbers cure quickly, though the rate of or even ability to cure is easily inhibited in the presence of elemental tin
Tin is a chemical element; it has symbol Sn () and atomic number 50. A silvery-colored metal, tin is soft enough to be cut with little force, and a bar of tin can be bent by hand with little effort. When bent, a bar of tin makes a sound, the ...
, sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
, and many amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
compounds.
Condensation cure system
''Condensation curing'' systems can be ''one-part'' or ''two-part'' systems. In one-part or ''RTV'' ( room-temperature vulcanizing) system, across-link
In chemistry and biology, a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
er exposed to ambient humidity (i.e., water) experiences a hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
step and is left with a hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
or silanol
A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon c ...
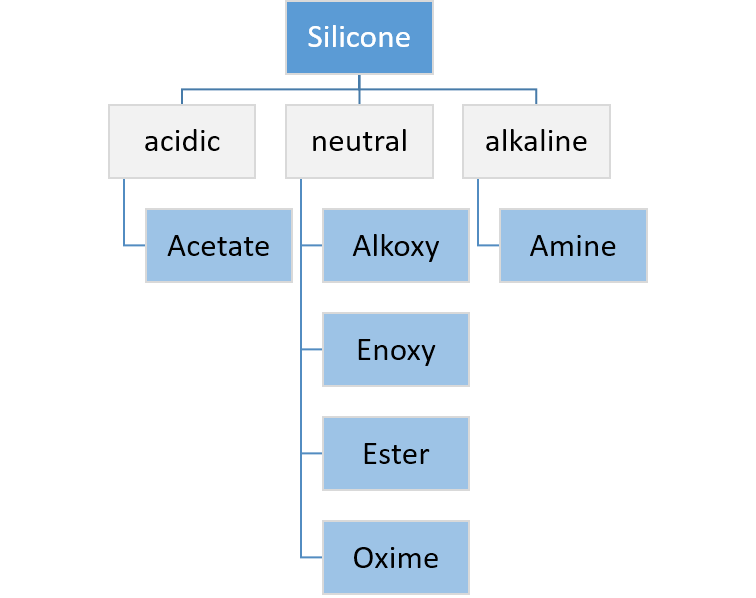
group. The silanol condenses further with another hydrolyzable group on the polymer or cross-linker and continues until the system is fully cured. Such a system will cure on its own at room temperature and (unlike the platinum-based addition cure system) is not easily inhibited by contact with other chemicals, though the process may be affected by contact with some plastics or metals and may not take place at all if placed in contact with already-cured silicone compounds. The crosslinkers used in condensation cure systems are typically alkoxy, acetoxy, ester, enoxy, or oxime silanes such as methyl trimethoxy silane for alkoxy-curing systems and methyl triacetoxysilane for acetoxy-curing systems. In many cases an additional condensation catalyst is added to fully cure the RTV system and achieve a tack-free surface. Organotitanate catalysts such as tetraalkoxy titanates or chelated titanates are used in alkoxy-cured systems. Tin catalysts such as dibutyl tin dilaurate (DBTDL) can be used in oxime and acetoxy-cured systems. Acetoxy tin condensation is one of the oldest cure chemistries used for curing silicone rubber, and is the one used in household bathroom caulk
Caulk (also known as caulking and calking) is a material used to Seal (mechanical), seal Joint (building), joints or seams against leakage in various structures and piping.
The oldest form of caulk consisted of fibrous materials driven into ...
. Depending on the type of detached molecule, it is possible to classify silicone systems as acidic, neutral, or alkaline.
Peroxide cure system
Peroxide curing is widely used for curing silicone rubber. The curing process leaves behind byproducts, which can be an issue in food contact and medical applications. However, these products are usually treated in a postcure oven which greatly reduces the peroxide breakdown product content. One of the two mainorganic peroxide
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily b ...
s used, dicumyl peroxide, has principal breakdown products of acetophenone
Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.
Production
Acetophenone is formed as a byproduct of the cumene ...
and 2-phenyl-2-propanol. The other is 2,4-dichlorobenzoyl peroxide, whose principal breakdown products are 2,4-dichlorobenzoic acid and 1,3-dichlorobenzene.

History
The first siliconeelastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of ''ela ...
s were developed in the search for better insulating materials for electric motors and generators. Resin-impregnated glass fibers were the state-of-the-art materials at the time. The glass was very heat resistant, but the phenolic resins would not withstand the higher temperatures that were being encountered in new smaller electric motors. Building on the work of Frederic Kipping
(Frederic) Stanley Kipping FRS (16 August 1863 – 1 May 1949) was an English chemist. He undertook much of the pioneering work on silicon polymers and coined the term silicone.
Life
He was born in Salford, Lancashire, England, the son of Jame ...
, chemists at Corning Glass and General Electric
General Electric Company (GE) was an American Multinational corporation, multinational Conglomerate (company), conglomerate founded in 1892, incorporated in the New York (state), state of New York and headquartered in Boston.
Over the year ...
were investigating heat-resistant materials for use as resinous binders when they synthesized the first silicone polymers, demonstrated that they worked well and found a route to produce them commercially.
Corning Glass in a joint venture with Dow Chemical
The Dow Chemical Company is an American multinational corporation headquartered in Midland, Michigan, United States. The company was among the three largest chemical producers in the world in 2021. It is the operating subsidiary of Dow Inc., ...
formed Dow Corning
Dow Corning Corporation, was an American multinational corporation headquartered in Midland, Michigan, United States, and was originally established as a joint venture between The Dow Chemical Company and Corning Inc., Corning Incorporated. In 20 ...
in 1943 to produce this new class of materials. War-time uses included gaskets for the B29's superchargers and supporting searchlight
A searchlight (or spotlight) is an apparatus that combines an extremely luminosity, bright source (traditionally a carbon arc lamp) with a mirrored parabolic reflector to project a powerful beam of light of approximately parallel rays in a part ...
lenses.
As the unique properties of the new silicone products were studied in more detail, their potential for broader usage was envisioned, and GE opened its own plant to produce silicones in 1947. GE Silicones was sold to Momentive Performance Materials in 2006. Wacker Chemie also started production of silicones in Europe in 1947. The Japanese company Shin-Etsu Chemical began mass production of silicone in 1953.
Properties
Silicone rubber offers good resistance to extreme temperatures, operating normally from . Silicone rubber has low tensile strength and poor wear-and-tear properties. Some properties such as elongation, creep, cyclic flexing, tear strength, compression set,dielectric strength
In physics, the term dielectric strength has the following meanings:
*for a pure electrically insulating material, the maximum electric field that the material can withstand under ideal conditions without undergoing electrical breakdown and becom ...
(at high voltage), thermal conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1.
Heat transfer occurs at a lower rate in materials of low ...
, fire resistance, and, in some cases, tensile strength
Ultimate tensile strength (also called UTS, tensile strength, TS, ultimate strength or F_\text in notation) is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials, the ultimate ...
can be—at extreme temperatures—far superior to organic rubbers in general, although a few of these properties are still lower than for some specialty materials. Silicone rubber is a material of choice in industry when retention of initial shape and mechanical strength are desired under heavy thermal stress or sub-zero temperatures.
Compared to organic rubber
Organic rubber has a carbon-to-carbon backbone which can leave it susceptible toozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
, UV, heat, and other aging factors that silicone rubber can withstand well. This makes silicone rubber one of the elastomers of choice in many extreme environments. Silicone is considerably more permeable to gasses than most other rubbers which limits its use in some areas.
Silicone rubber is highly inert, does not react with most chemicals, and does not participate in biological processes, allowing it to be used in many medical applications including medical implants.
It is biocompatible and hypoallergenic
Hypoallergenic, meaning "below average" or "slightly" allergenic, is a term meaning that something (usually cosmetics, pets, textiles, food, etc.) causes fewer allergic reactions. The term was first used in 1953 in an advertising campaign for co ...
, which makes it suitable for baby care products, and food contact in general.
Silicone rubber is a reliable solution (as opposed to rubber and thermoplastic elastomer
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers (TPR), are a class of copolymers or a physical mix of polymers (usually a plastic and a rubber) that consist of materials with both thermoplastic and elastomeric prop ...
s) for migration or interaction problems between the main active ingredients. Its chemical stability prevents it from affecting any substrate it is in contact with (skin, water, blood, active ingredients, etc.).
:
Production
To make silicone, the silicon atoms must be isolated from the silicon dioxide compoundsilica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
. This is done by heating large volumes of quartz sand to extremely high temperatures, often up to 1800 °C. From here, there are several processes where silicon is combined with methyl chloride
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in indus ...
and heated. It is then distilled into a polymerised siloxane
In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: . The parent siloxanes include the oligomeric and polymeric hydrides with the formulae and . Siloxanes ...
known as polydimethylsiloxane
Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication and passive daytime radiative cooling.
PDMS is particularly known for its ...
. The polydimethylsiloxane can then be polymerised. This is done using a variety of techniques depending on the use of the final product. The raw silicone compound is combined with any desired additives, which may include pigments, and the catalyst. It is then injection moulded, extruded, or 3D printed. Curing is the final stage in the production process.
Structure
polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bott ...
. A C–C backbone unit has a bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
of 1.54 Å and a bond angle of 112°, whereas an Si–O backbone unit has a bond length of 1.63 Å and a bond angle of 130°. Polymer segments in polysiloxanes can move farther and change conformation easily, making for a flexible material. Polysiloxanes tend to be more stable and less chemically active because more energy is required to break the silicon–oxygen bond. Although silicon is a congener of carbon, having the same electron bonding configuration, silicon analogues of carbonaceous compounds generally exhibit different properties. The difference in total charge and mass between carbon with 6 protons and 6 neutrons, and silicon with 14 protons and 14 neutrons causes an added layer of electrons and their screening effect changes the electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
between the two elements. For example the silicon–oxygen bond in polysiloxanes is significantly more stable than the carbon-oxygen bond in polyoxymethylene
Polyoxymethylene (POM), also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts requiring high stiffness, low friction, and excellent dimensional stability. Short-chained POM (chain length ...
, a structurally similar polymer. The difference is partly due to the higher bond energy
In chemistry, bond energy (''BE'') is one measure of the strength of a chemical bond. It is sometimes called the mean bond, bond enthalpy, average bond enthalpy, or bond strength. IUPAC defines bond energy as the average value of the gas-phase b ...
, the energy required to break the Si-O bond, and also because polyoxymethylene decomposes formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ...
, which is volatile and escapes driving decomposition forward, but Si-containing decomposition products of silicone are less volatile.
:
Special grades
There are many special grades and forms of silicone rubber, including:steam
Steam is water vapor, often mixed with air or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Saturated or superheated steam is inv ...
resistant, metal detectable, high tear strength, extreme high temperature, extreme low temperature, electrically conductive
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
, chemical/oil/acid/gas resistant, low smoke emitting, optically clear, and flame-retardant. A variety of fillers can be used in silicone rubber, although most are non-reinforcing and lower the tensile strength
Ultimate tensile strength (also called UTS, tensile strength, TS, ultimate strength or F_\text in notation) is the maximum stress that a material can withstand while being stretched or pulled before breaking. In brittle materials, the ultimate ...
.
Silicone rubber is available in a range of hardness levels, expressed as Shore A or IRHD between 10 and 100, the higher number being the harder compound. It is also available in virtually any colour, and can be colour matched.
Applications
Silicone rubber is used in automotive applications, many cooking, baking, and food storage products, apparel including undergarments, sportswear, and footwear, electronics, to home repair and hardware, and a host of unseen applications. It is usually processed and shaped with the following methods.Extrusion
Once mixed and coloured, silicone rubber can beextruded
Extrusion is a process used to create objects of a fixed cross-sectional profile by pushing material through a die of the desired cross-section. Its two main advantages over other manufacturing processes are its ability to create very complex ...
into tubes, strips, solid cord, or custom profiles according to the size specifications of the manufacturer. Cord can be joined to make O-ring
An O-ring, also known as a packing or a toric joint, is a mechanical gasket in the shape of a torus; it is a loop of elastomer with a round cross section (geometry), cross-section, designed to be seated in a groove and compressed during assembl ...
s and extruded profiles can be joined to make seals.
Injection moulding
3D printing
pot life
Pot may refer to:
Containers
* Flowerpot, a container in which plants are cultivated
* Pottery, ceramic containers made from clay
* Cooking pot, a type of cookware
* Pot, a beer glass
Places
* Ken Jones Aerodrome, IATA airport code POT
* ...
need to be adjusted.
3D printing also requires the use of a removable support material that is compatible with the silicone rubber.
Liquid silicone rubber is also manufactured for life science
Life, also known as biota, refers to matter that has biological processes, such as signaling and self-sustaining processes. It is defined descriptively by the capacity for homeostasis, organisation, metabolism, growth, adaptation, respon ...
applications (syringe pistons, closure for dispensing system, gaskets for IV flow regulator, respiratory masks, and implantable chambers for IV administration), cosmetic products (mascara brush, make-up packaging, make-up applicator, and lipstick moulds), and optics products (circular lens, collimator
A collimator is a device which narrows a beam of particles or waves. To narrow can mean either to cause the directions of motion to become more aligned in a specific direction (i.e., make collimated light or parallel rays), or to cause the spat ...
s, Fresnel lens
A Fresnel lens ( ; ; or ) is a type of composite compact lens (optics), lens which reduces the amount of material required compared to a conventional lens by dividing the lens into a set of concentric annular sections.
The simpler Dioptrics, d ...
es, and free-form lenses).
Freeze-tolerant solar water-heating panels exploit the elasticity of silicone to repeatedly accommodate the expansion of water on freezing, while its extreme temperature tolerance maintains a lack of brittleness below freezing and excellent tolerance of temperatures in excess of . Its property of not having a carbon backbone, but a chemically robust silicon backbone instead, reduces its potential as a food source for dangerous waterborne bacteria such as Legionella
''Legionella'' is a genus of gram-negative bacteria, gram-negative bacteria that can be seen using a silver stain or grown in a special media that contains cysteine, an amino acid. It is known to cause legionellosis (all illnesses caused by ''Legi ...
.
Non-dyed silicone rubber tape with an iron(III) oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula . It occurs in nature as the mineral hematite, which serves as the primary source of iron for the steel industry. It is also known as red iron oxide, especially when use ...
additive (making the tape a red-orange colour) is used extensively in aviation and aerospace wiring applications as a splice or wrapping tape due to its non-flammable nature. The iron oxide additive adds high thermal conductivity but does not change the high electrical insulation property of the silicone rubber. This type of self-amalgamating tape amalgamates or fuses to itself, so that when stretched and wrapped around cables, electrical joints, hoses, and pipes it bonds into a strong seamless rubbery electrically insulating and waterproof layer, although not adhesive.
As an electrical insulator, silicone rubber has the added virtue of remaining non-conductive when damaged by heat, reducing the likelihood of runaway arcing.
With the addition of carbon or another conductive substance as a powdered filler, silicone rubber can be made electrically conductive while retaining most of its other mechanical properties. As such it is used for flexible contacts which close on being pressed, used in many devices such as computer keyboard
A computer keyboard is a built-in or peripheral input device modeled after the typewriter keyboard which uses an arrangement of buttons or Push-button, keys to act as Mechanical keyboard, mechanical levers or Electronic switching system, electro ...
s and remote control
A remote control, also known colloquially as a remote or clicker, is an consumer electronics, electronic device used to operate another device from a distance, usually wirelessly. In consumer electronics, a remote control can be used to operat ...
handsets.
Electrical insulation
Silicone rubber is used as anelectrical insulator
An electrical insulator is a material in which electric current does not flow freely. The atoms of the insulator have tightly bound electrons which cannot readily move. Other materials—semiconductors and electrical conductor, conductors—con ...
in power cable
A power cable is an electrical cable used specifically for transmission of electric energy, electrical power. It is an assembly of one or more electrical conductors, usually held together in a single bundle with an insulator (electricity), insu ...
s and cable joints. Silicone-insulated cables are advantageous in that they can withstand temperatures from -90°C to 200°C, and are highly flexible. These properties make them suitable for maintaining circuit integrity in the event of a fire.
Self-healing
In 2007, silicone rubber formed the matrix of the first autonomic self-healing elastomer.Keller ''et al.'', ''A Self-Healing Poly(dimethyl siloxane) Elastomer,'' Advanced Functional Materials, v. 17, p. 2399–2404 (2007). The microcapsule-based material was capable of recovering almost all of the original tear strength. Additionally, this material had improved fatigue properties as evaluated using a torsion-fatigue test.Keller ''et al.'', ''Torsion Fatigue Response of Self-Healing Poly(dimethyl siloxane) Elastomers'', Polymer, v.49 p. 3136–3145 (2008).See also
*Forensic engineering
Forensic engineering has been defined as "the investigation of failures—ranging from serviceability to catastrophic—which may lead to legal activity, including both civil and criminal". The forensic engineering field is very broad in terms o ...
* Forensic polymer engineering
* Injection molding of liquid silicone rubber
* Medical grade silicone
* RTV silicone
RTV silicone (room-temperature- vulcanizing silicone) is a type of silicone rubber that cures at room temperature. It is available as a one-component product, or mixed from two components (a base and curative). Manufacturers provide it in a range o ...
References
Further reading
* Brydson, John (1999) ''Plastics Materials'', Butterworth, 9th Ed * Lewis, PR, Reynolds, K and Gagg, C (2004) ''Forensic Materials Engineering: Case Studies'', CRC Press {{Rubber Elastomers Sculpture materials * * de:Silikone#Silikonkautschuk zh:硅橡胶