selective permeability on:
[Wikipedia]
[Google]
[Amazon]
Semipermeable membrane is a type of synthetic or biologic,

The European Membrane House
a non-profit international association created to continue the work of the network and partnerships developed in NanoMemPro, an earlier EU-funded European network of membrane researchers.
Short, non-scholarly WiseGeek article, "What is a Semipermeable Membrane.
{{Galvanic cells Diffusion Filters Membrane biology Membrane technology
polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
ic membrane that allows certain molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s or ions to pass through it by osmosis. The rate of passage depends on the pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
, concentration, and temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg.
Biological membranes are selectively permeable, with the passage of molecules controlled by facilitated diffusion, passive transport or active transport regulated by proteins embedded in the membrane.
Biological membranes
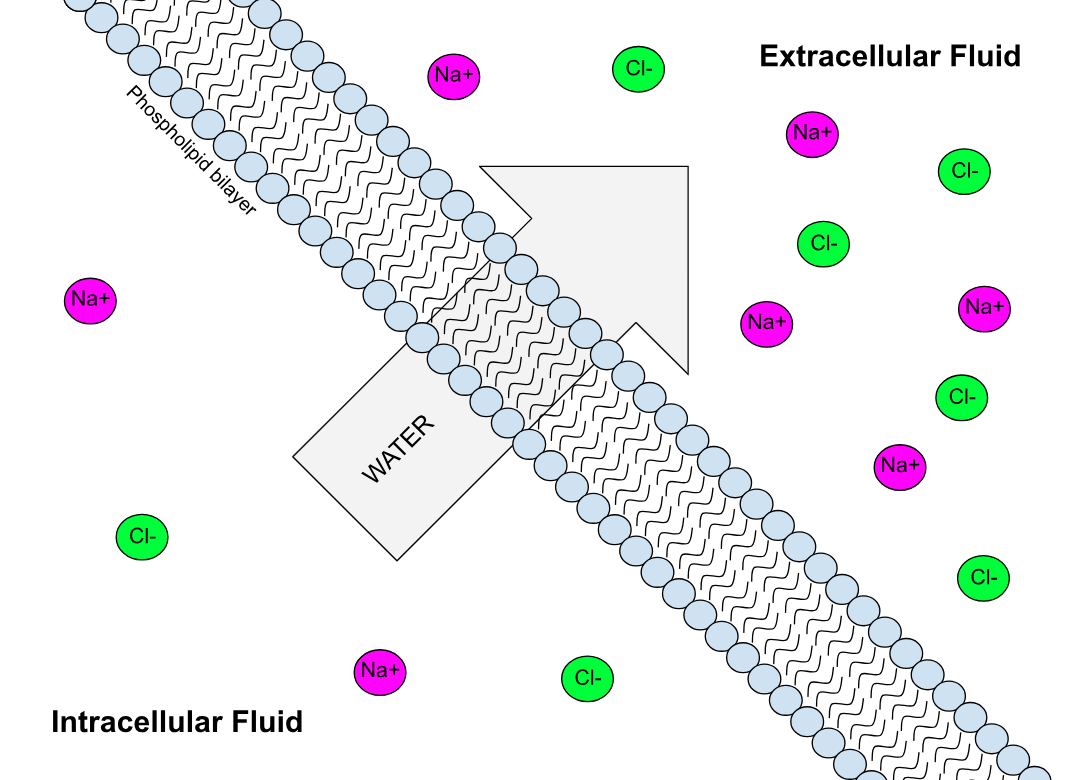
Phospholipid bilayer
A phospholipid bilayer is an example of a biological semipermeable membrane. It consists of two parallel, opposite-facing layers of uniformly arrangedphospholipid
Phospholipids are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s. Each phospholipid is made of one phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
head and two fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
tails. The plasma membrane that surrounds all biological cells is an example of a phospholipid bilayer. The plasma membrane is very specific in its permeability, meaning it carefully controls which substances enter and leave the cell. Because they are attracted to the water content within and outside the cell (or ''hydrophillic''), the phosphate heads assemble along the outer and inner surfaces of the plasma membrane, and the hydrophobic tails are the layer hidden in the inside of the membrane. Cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
molecules are also found throughout the plasma membrane and act as a buffer of membrane fluidity. The phospholipid bilayer is most permeable to small, uncharged solutes. Protein channels are embedded in or through the phospholipids, and, collectively, this model is known as the fluid mosaic model. Aquaporins are protein channel pores permeable to water.
Cellular communication
Information can also pass through the plasma membrane when signaling molecules bind to receptors in the cell membrane. The signaling molecules bind to the receptors, which alters the structure of these proteins. A change in the protein structure initiates a signaling cascade. G protein-coupled receptor signaling is an important subset of such signaling processes.
Osmotic stress
Because the lipid bilayer is semipermeable, it is subject to osmotic pressure. When the solutes around a cell become more or less concentrated, osmotic pressure causes water to flow into or out of the cell to equilibrate. This osmotic stress inhibits cellular functions that depend on the activity of water in the cell, such as the functioning of its DNA and protein systems and proper assembly of its plasma membrane. This can lead to osmotic shock and cell death. Osmoregulation is the method by which cells counteract osmotic stress, and includes osmosensory transporters in the membrane that allow K+ and othermolecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s to flow through the membrane.
Artificial membranes
Artificial semipermeable membranes see wide usage in research and the medical field. Artificial lipid membranes can easily be manipulated and experimented upon to study biological phenomenon. Other artificial membranes include those involved in drug delivery, dialysis, and bioseparations.Reverse osmosis
The bulk flow of water through a selectively permeable membrane because of an osmotic pressure difference is called osmosis. This allows only certain particles to go through including water and leaving behind the solutes including salt and other contaminants. In the process of reverse osmosis, water is purified by applying high pressure to a solution and thereby push water through a thin-film composite membrane (TFC or TFM). These are semipermeable membranes manufactured principally for use in water purification or desalination systems. They also have use in chemical applications such as batteries and fuel cells. In essence, a TFC material is a molecular sieve constructed in the form of a film from two or more layered materials. Sidney Loeb and Srinivasa Sourirajan invented the first practical synthetic semi-permeable membrane. Membranes used in reverse osmosis are, in general, made out of polyamide, chosen primarily for its permeability to water and relative impermeability to various dissolved impurities including salt ions and other small molecules that cannot be filtered.Regeneration of reverse osmosis membranes
Reverse osmosis membrane modules have a limited life cycle, several studies have endeavored to improve the performance of the process and extend the RO membranes lifespan. However, even with the appropriate pretreatment of the feed water, the membranes lifespan is generally limited to five to seven years. Discarded RO membrane modules are currently classified worldwide as inert solid waste and are often disposed of in landfills, with limited reuse. Estimates indicated that the mass of membranes annually discarded worldwide reached 12,000 tons. At the current rate, the disposal of RO modules represents significant and growing adverse impacts on the environment, giving rise to the need to limit the direct discarding of these modules. Discarded RO membranes from desalination operations could be recycled for other processes that do not require the intensive filtration criteria of desalination, they could be used in applications requiring nanofiltration (NF) membranes. Regeneration process steps: 1- Chemical Treatment Chemical procedures aimed at removing fouling from the spent membrane; several chemicals agents are used; such as: - Sodium Hydroxide (alkaline) - Hydrochloric Acid (Acidic) - Chelating agents Such as Citric and Oxalic acids There are three forms of membranes exposure to chemical agents; simple immersion, recirculating the cleaning agent, or immersion in an ultrasound bath. 2 - Oxidative treatment It includes exposing the membrane to oxidant solutions in order to remove its dense aromatic polyamide active layer and subsequent conversion to a porous membrane. Oxidizing agents such as Sodium Hypochlorite NaClO (10–12%) and Potassium Permanganate KMnO₄ are used. These agents remove organic and biological fouling from RO membranes, They also disinfect the membrane surface, preventing the growth of bacteria and other microorganisms. Sodium Hypochlorite is the most efficient oxidizing agent in light of permeability and salt rejection solution.
Dialysis tubing
Dialysis tubing is used in hemodialysis to purify blood in the case of kidney failure. The tubing uses a semipermeable membrane to remove waste before returning the purified blood to the patient. Differences in the semipermeable membrane, such as size of pores, change the rate and identity of removed molecules. Traditionally,cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important s ...
membranes were used, but they could cause inflammatory responses in patients. Synthetic membranes have been developed that are more biocompatible and lead to fewer inflammatory responses. However, despite the increased biocompatibility, synthetic membranes have not been linked to decreased mortality.
Other types
Other types of semipermeable membranes are cation-exchange membranes (CEMs), anion-exchange membranes (AEMs), alkali anion-exchange membranes (AAEMs) and proton-exchange membranes (PEMs).Notes
References
Further reading
* See this document for definitions of penetrant (permeant), synthetic (artificial) membrane, and anion-exchange membrane. * *External links
The European Membrane House
a non-profit international association created to continue the work of the network and partnerships developed in NanoMemPro, an earlier EU-funded European network of membrane researchers.
Short, non-scholarly WiseGeek article, "What is a Semipermeable Membrane.
{{Galvanic cells Diffusion Filters Membrane biology Membrane technology