Sandmeyer Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Sandmeyer reaction is a  The most commonly employed Sandmeyer reactions are the chlorination, bromination, cyanation, and hydroxylation reactions using CuCl, CuBr, CuCN, and Cu2O, respectively. More recently, trifluoromethylation of diazonium salts has been developed and is referred to as a 'Sandmeyer-type' reaction. Diazonium salts also react with boronates, iodide,
The most commonly employed Sandmeyer reactions are the chlorination, bromination, cyanation, and hydroxylation reactions using CuCl, CuBr, CuCN, and Cu2O, respectively. More recently, trifluoromethylation of diazonium salts has been developed and is referred to as a 'Sandmeyer-type' reaction. Diazonium salts also react with boronates, iodide,

 There are many synthetic applications of the Sandmeyer reaction.
There are many synthetic applications of the Sandmeyer reaction.
 One bromination protocol employs a Cu(I)/Cu(II) mixture with additional amounts of the bidentate
One bromination protocol employs a Cu(I)/Cu(II) mixture with additional amounts of the bidentate 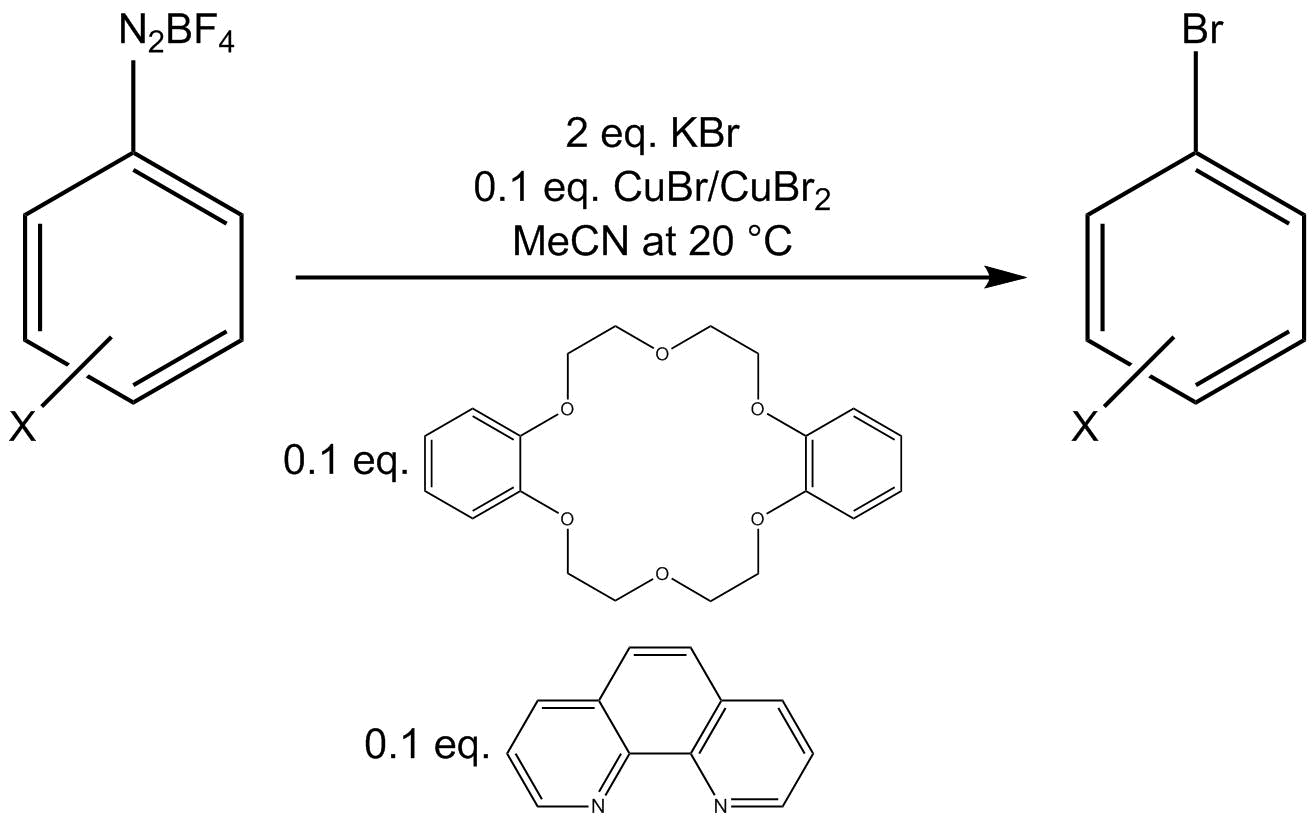 The
The
 The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target
The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target 


chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
used to synthesize aryl halide
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, br ...
s from aryl diazonium salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, comp ...
s using copper salts as reagents or catalysts.
It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides a method through which one can perform unique transformations on benzene, such as halogenation
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drug ...
, cyanation, trifluoromethylation Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notab ...
, and hydroxylation
In chemistry, hydroxylation refers to the installation of a hydroxyl group () into an organic compound. Hydroxylations generate alcohols and phenols, which are very common functional groups. Hydroxylation confers some degree of water-solubility ...
.
The reaction was discovered in 1884 by Swiss chemist Traugott Sandmeyer, when he attempted to synthesize phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas.
Preparation
In ...
from benzenediazonium chloride
Benzenediazonium tetrafluoroborate is an organic compound with the formula 6H5N2F4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the ary ...
and copper(I) acetylide
Copper(I) acetylide, copper carbide or cuprous acetylide, is a chemical compound with the formula . It is a copper(I) salt of acetylene. It consists of cations and acetylide anions , with the triple bond between the two carbon atoms. Although nev ...
. Instead, the main product he isolated was chlorobenzene
Chlorobenzene (abbreviated PhCl) is an aryl chloride and the simplest of the chlorobenzenes, consisting of a benzene ring substituted with one chlorine atom. Its chemical formula is C6H5Cl. This colorless, flammable liquid is a common solvent a ...
. In modern times, the Sandmeyer reaction refers to any method for substitution of an aromatic amino group via preparation of its diazonium salt followed by its displacement with a nucleophile in the presence of catalytic copper(I) salts.
: The most commonly employed Sandmeyer reactions are the chlorination, bromination, cyanation, and hydroxylation reactions using CuCl, CuBr, CuCN, and Cu2O, respectively. More recently, trifluoromethylation of diazonium salts has been developed and is referred to as a 'Sandmeyer-type' reaction. Diazonium salts also react with boronates, iodide,
The most commonly employed Sandmeyer reactions are the chlorination, bromination, cyanation, and hydroxylation reactions using CuCl, CuBr, CuCN, and Cu2O, respectively. More recently, trifluoromethylation of diazonium salts has been developed and is referred to as a 'Sandmeyer-type' reaction. Diazonium salts also react with boronates, iodide, thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s, water, hypophosphorous acid
Hypophosphorous acid (HPA), or phosphinic acid, is a phosphorus oxyacid and a powerful reducing agent with molecular formula H3PO2. It is a colorless low-melting compound, which is soluble in water, dioxane
and alcohols. The formula for th ...
and others, and fluorination can be carried out using tetrafluoroborate anions (Balz–Schiemann reaction
The Balz–Schiemann reaction (also called the Schiemann reaction) is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to ...
). However, since these processes do not require a metal catalyst, they are not usually referred to as Sandmeyer reactions. In numerous variants that have been developed, other transition metal salts, including copper(II), iron(III) and cobalt(III) have also been employed. Due to its wide synthetic applicability, the Sandmeyer reaction, along with other transformations of diazonium compounds, is complementary to electrophilic aromatic substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitut ...
.
Reaction mechanism
The Sandmeyer reaction is an example of a radical-nucleophilic aromatic substitution (SRNAr). The radical mechanism of the Sandmeyer reaction is supported by the detection of biaryl byproducts. The substitution of the aromatic diazo group with a halogen orpseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorgani ...
is initiated by a one-electron transfer mechanism catalyzed by copper(I) to form an aryl radical An aryl radical in organic chemistry is a reactive intermediate and an arene compound incorporating one free radical carbon atom as part of the ring structure. As such it is the radical counterpart of the arenium ion. The parent compound is the ...
with loss of nitrogen gas. The substituted arene
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
is possibly formed by direct transfer of Cl, Br, CN, or OH from a copper(II) species to the aryl radical to produce the substituted arene and regenerate the copper(I) catalyst. In an alternative proposal, a transient copper(III) intermediate, formed from coupling of the aryl radical with the copper(II) species, undergoes rapid reductive elimination to afford the product and regenerate copper(I). However, evidence for such an organocopper intermediate is weak and mostly circumstantial, and the exact pathway may depend on the substrate and reaction conditions.
Single electron transfer

Synthetic applications
Variations on the Sandmeyer reaction have been developed to fit multiple synthetic applications. These reactions typically proceed through the formation of an aryl diazonium salt followed by a reaction with a copper(I) salt to yield a substituted arene:Halogenation
One of the most important uses of the Sandmeyer reaction is the formation of aryl halides. The solvent of choice for the synthesis ofiodoarenes
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bro ...
is diiodomethane
Diiodomethane or methylene iodide, commonly abbreviated "MI", is an organoiodine compound. Diiodomethane is a very dense colorless liquid; however, it decomposes upon exposure to light liberating iodine, which colours samples brownish. It is slig ...
, while for the synthesis of bromoarenes
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bro ...
, bromoform
Bromoform is an organic compound with the chemical formula . It is a colorless liquid at room temperature, with a high refractive index and a very high density. Its sweet odor is similar to that of chloroform. It is one of the four haloforms, the ...
is used. For the synthesis of chloroarenes
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bro ...
, chloroform
Chloroform, or trichloromethane (often abbreviated as TCM), is an organochloride with the formula and a common solvent. It is a volatile, colorless, sweet-smelling, dense liquid produced on a large scale as a precursor to refrigerants and po ...
is the solvent of choice. The synthesis of (+)- curcuphenol, a bioactive compound that displays antifungal and anticancer activity, employs the Sandmeyer reaction to substitute an amine group by a bromo group.
 One bromination protocol employs a Cu(I)/Cu(II) mixture with additional amounts of the bidentate
One bromination protocol employs a Cu(I)/Cu(II) mixture with additional amounts of the bidentate ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
phenanthroline
1,10-Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. The 1,10 refers to the location of the nitrogen atoms that replace CH's in the hydrocarbon called phenanthrene.
Abbreviated " ...
and phase-transfer catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the Phase transition, transition of a reactant from one phase (matter), phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of cat ...
dibenzo-18-crown-6 to convert an aryl diazonium tetrafluoroborate salt to an aryl bromide.
: The
The Balz–Schiemann reaction
The Balz–Schiemann reaction (also called the Schiemann reaction) is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to ...
uses tetrafluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perc ...
and delivers the halide-substituted product, fluorobenzene
Fluorobenzene is an aryl fluoride and the simplest of the fluorobenzenes, with the formula C6H5F, often abbreviated Phenyl group, PhF. A colorless liquid, it is a precursor to many fluorophenyl compounds.
Preparation
PhF was first reported in 18 ...
, which is not obtained by the use of copper fluoride Copper fluoride may refer to:
* Copper(I) fluoride (cuprous fluoride, CuF).
*Copper(II) fluoride
Copper(II) fluoride or cupric fluoride is an inorganic compound with the chemical formula CuF2. The anhydrous form is a white, ionic, crystalline, ...
s. This reaction displays motifs characteristic of the Sandmeyer reaction.
Cyanation
Another use of the Sandmeyer reaction is for cyanation which allows for the formation ofbenzonitrile
Benzonitrile is the chemical compound with the formula , abbreviated PhCN. This aromatic organic compound is a colorless liquid with a cherry or almond like odour. It is mainly used industrially to produce the melamine resin precursor benzoguanam ...
s, an important class of organic compounds. A key intermediate in the synthesis of the antipsychotic drug Fluanxol is synthesized by a cyanation through the Sandmeyer reaction.
 The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target
The Sandmeyer reaction has also been employed in the synthesis of neoamphimedine, a compound that is suggested to target topoisomerase II
Type II topoisomerases are topoisomerases that cut both strands of the DNA helix simultaneously in order to manage DNA tangles and supercoils. They use the hydrolysis of Adenosine triphosphate, ATP, unlike Type I topoisomerase. In this process, t ...
as an anti-cancer drug.

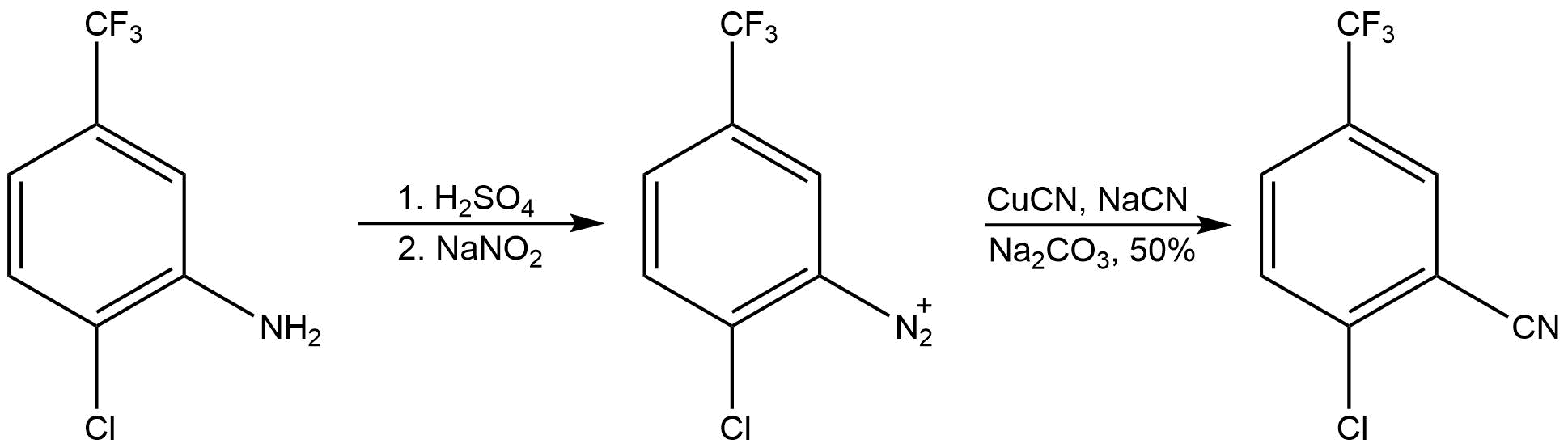
Trifluoromethylation
It has been demonstrated that Sandmeyer-type reactions can be used to generate aryl compounds functionalized by trifluoromethyl substituent groups. This process oftrifluoromethylation Trifluoromethylation in organic chemistry describes any organic reaction that introduces a trifluoromethyl group in an organic compound. Trifluoromethylated compounds are of some importance in pharmaceutical industry and agrochemicals. Several notab ...
provides unique chemical properties with a wide variety of practical applications. Particularly, pharmaceuticals with CF3 groups have enhanced metabolic stability
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of ...
, lipophilicity
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly") is the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such compounds are called lipophilic (translated ...
, and bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. H ...
. Sandmeyer-type trifluoromethylation reactions feature mild reaction conditions and greater functional group tolerance relative to earlier methods of trifluoromethylation. An example of a Sandmeyer-type trifluoromethylation reaction is presented below.

Hydroxylation
The Sandmeyer reaction can also be used to convert aryl amines tophenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
s proceeding through the formation of an aryl diazonium salt. In the presence of copper catalyst, such as copper(I) oxide
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula . It is one of the principal oxides of copper, the other being copper(II) oxide or cupric oxide (CuO). The compound can appear either yellow or red, depending on the size ...
, and an excess of copper(II) nitrate
Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu( NO3)2(H2O)x. The hydrates are hygroscopic blue solids. Anhydrous copper nitrate forms blue-green crystals and sublimes in a vacuum at 150-200 ° ...
, this reaction takes place readily at room temperature neutral water. This is in contrast to the classical procedure (known by the German name '), which calls for boiling the diazonium salt in aqueous acid, a process that is believed to involve the aryl cation instead of radical and is known to generate other nucleophilic addition side products in addition to the desired hydroxylation product.

References
External links
* http://www.name-reaction.com/sandmeyer-reaction {{DEFAULTSORT:Sandmeyer Reaction Substitution reactions Name reactions