safety syringe on:
[Wikipedia]
[Google]
[Amazon]
A safety syringe is a 
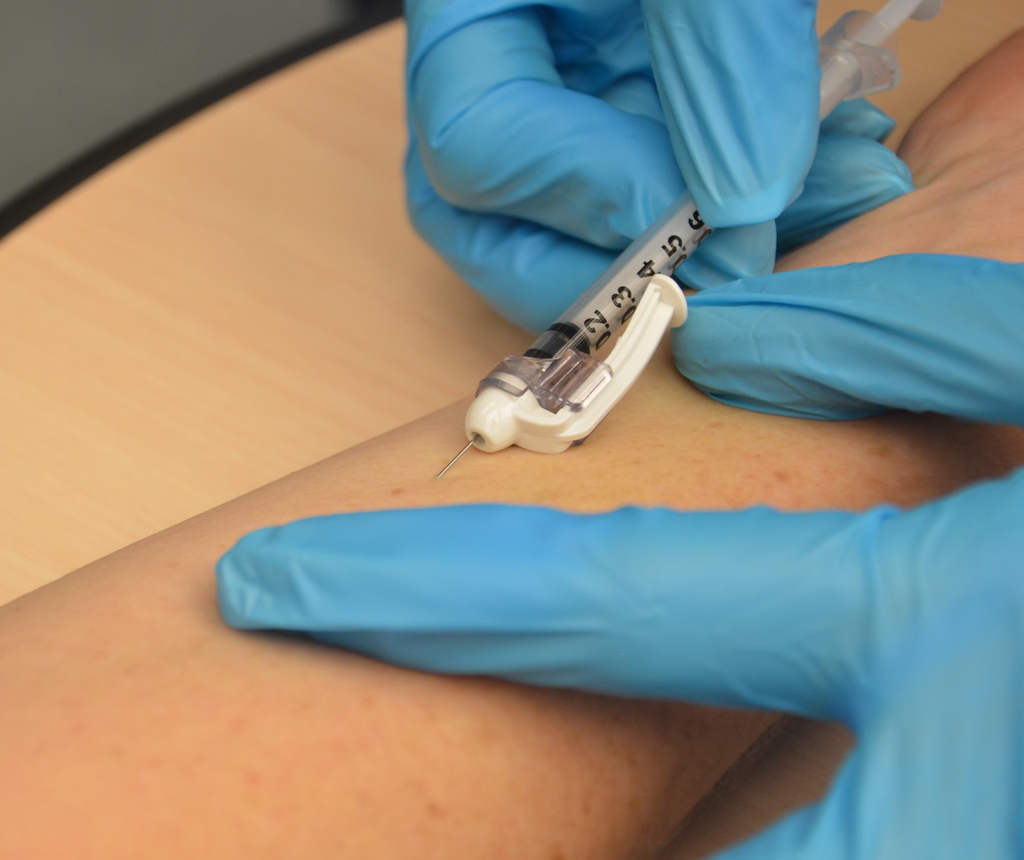 The more effective safety syringes have reuse and needlestick prevention features. A sheath or hood slides over the needle after the injection is completed with a Needlestick Prevention Syringe, which also has a re-use prevention feature (either an auto disable mechanism or breaking plunger). Retractable syringes use either manual or spring-loaded retraction to withdraw the needle into the barrel of the syringe. Some brands of spring-loaded syringes can have a splatter effect, where blood and fluids are sprayed off the
The more effective safety syringes have reuse and needlestick prevention features. A sheath or hood slides over the needle after the injection is completed with a Needlestick Prevention Syringe, which also has a re-use prevention feature (either an auto disable mechanism or breaking plunger). Retractable syringes use either manual or spring-loaded retraction to withdraw the needle into the barrel of the syringe. Some brands of spring-loaded syringes can have a splatter effect, where blood and fluids are sprayed off the 
W.H.O. Injection Safety Toolbox
W.H.O. Injection Safety
Centers for Disease Control – Injection Safety
• Washington Monthly, Jul/Aug 2010,
Medical equipment Drug delivery devices
syringe
A syringe is a simple reciprocating pump consisting of a plunger (though in modern syringes, it is actually a piston) that fits tightly within a cylindrical tube called a barrel. The plunger can be linearly pulled and pushed along the inside ...
with a built-in safety mechanism to reduce the risk of needlestick injuries to healthcare workers and others. The needle on a safety syringe can be detachable or permanently attached. On some models, a sheath is placed over the needle, whereas in others the needle retracts into the barrel. Safety needles serve the same functions as safety syringes, but the protective mechanism is a part of the needle rather than the syringe. Legislation requiring safety syringes or equivalents has been introduced in many nations since needlestick injuries and re-use prevention became the focus of governments and safety bodies.
Types
There are many types of safety syringes available on the market. Auto Disable (AD) syringes are designed as a single use syringe, with an internal mechanism blocking the barrel once depressed so it cannot be depressed again. The other type of syringe with a re-use prevention feature is the breaking plunger syringe. An internal mechanism cracks the syringe when the plunger is fully depressed to prevent further use. These syringes are only effectively disabled with a full depression of the plunger; users can avoid activating the re-use prevention feature and re-use the syringe. The more effective safety syringes have reuse and needlestick prevention features. A sheath or hood slides over the needle after the injection is completed with a Needlestick Prevention Syringe, which also has a re-use prevention feature (either an auto disable mechanism or breaking plunger). Retractable syringes use either manual or spring-loaded retraction to withdraw the needle into the barrel of the syringe. Some brands of spring-loaded syringes can have a splatter effect, where blood and fluids are sprayed off the
The more effective safety syringes have reuse and needlestick prevention features. A sheath or hood slides over the needle after the injection is completed with a Needlestick Prevention Syringe, which also has a re-use prevention feature (either an auto disable mechanism or breaking plunger). Retractable syringes use either manual or spring-loaded retraction to withdraw the needle into the barrel of the syringe. Some brands of spring-loaded syringes can have a splatter effect, where blood and fluids are sprayed off the cannula
A cannula (; Latin meaning 'little reed'; : cannulae or cannulas) is a tube that can be inserted into the body, often for the delivery or removal of fluid or for the gathering of samples. In simple terms, a cannula can surround the inner or out ...
from the force of the retraction. Manual retraction syringes are generally easier to depress because there is no resistance from a spring.

Alternatives
Traditional glass syringes can be re-used once disinfected. Plastic body syringes have become more popular in recent years because they are disposable. Unfortunately, improper disposal methods and re-use are responsible for transferring blood borne diseases. This issue arises often by accident due to medications being mixed or switched out with others. Data is collected through "Patient Notification" events whereby organizations inform patients of possible exposure. These organizations then offer bloodborne pathogen testing to affected individuals.Importance
Of the 55 cases documented by theCDC
The Centers for Disease Control and Prevention (CDC) is the national public health agency of the United States. It is a United States federal agency under the Department of Health and Human Services (HHS), and is headquartered in Atlanta, ...
of (non-sex work
Sex work is "the exchange of sexual services, performances, or products for material compensation. It includes activities of direct physical contact between buyers and sellers as well as indirect sexual stimulation". Sex work only refers to volun ...
) occupational transmission of HIV
The human immunodeficiency viruses (HIV) are two species of '' Lentivirus'' (a subgroup of retrovirus) that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the im ...
, 90% were from contaminated needles that pierced the skin. The direct cost of needlestick injuries was calculated in a recent study to be between $539 and $672 million US dollars. That includes only lab tests, treatment, service and "other"; it does not take into account lost time and wages for employers and individuals.
Legislation
United States
*Needlestick Safety and Prevention Act, effective date 2001 Two lawyers, Mike Weiss and Paul Danzinger, were approached in 1998 by an inventor, Thomas Shaw, who was having trouble selling a safety syringe developed to protect health care workers from accidentally being infected by dirty needles. The problems were due to monopolistic actions of a major industry needle maker and hospital group purchasing organizations. The case was settled before trial for $150 million. This was portrayed by the 2011 movie ''Puncture
Puncture, punctured or puncturing may refer to:
* a flat tyre in British English (US English "flat tire" or just "flat")
* a penetrating wound caused by pointy objects as nails or needles
* Lumbar puncture, also known as a spinal tap
* Puncture ( ...
''. Shaw's attempts to get his retractable needle accepted by health care facilities were covered in a 2010 ''Washington Monthly
''Washington Monthly'' is a bimonthly, nonprofit magazine primarily covering United States politics and government that is based in Washington, D.C. The magazine also publishes an annual ranking of American colleges and universities, which ser ...
'' article.
Canada
*Health Canada Laboratory Biosafety Guidelines *Provincial Legislation: **British Columbia **Alberta **Manitoba **Saskatchewan **Ontario **Nova ScotiaAustralia
*No nationwide legislation is in place, but suggested practices or policies have been implemented in New South Wales, Victoria, and Queensland. **New South Wales uses a set of guidelines called "Blood and Body Substances Occupational Exposure Prevention" which was revised in 2024. This guideline details best practices for prevention against needlestick injuries for those working in the healthcare field. It Was made in response to the "Worker Health and Safety Act of 2011" and the "Worker Health and Safety Regulation 2017". **Victoria uses a set of guidelines to provide instruction for workplace safety regarding needles and sharps. It elaborates on exposure protocols, evaluation and testing of the source, and management methods for immediate and follow-up actions to be taken. **Queensland has guidance for blood and body fluid exposure. This details chief responsibilities, first aid, risk assessment, testing, among other topics regarding bloodborne pathogen exposure and management. Unlike the guidance from the other states, Queensland has more information regarding neonatal and pediatric considerations.Europe
*The European Union has some regulations on this subject. One of these regulations is that of "Conformity Assessments". These are tests conducted by "Notified bodies" and approved by the EU to evaluate performance and safety of the product. Depending on the specific device used, it may be considered a "High Risk" device. If a device is deemed "High Risk", then an additional panel of experts will be needed in the approval process.Sharps Containers
Sharps containers are devices used to collect small, sharp objects safely. The purpose of this is to properly dispose of contaminated items to limit exposure to workers nearby. These items often include needles, lancets, suture needles, and scalpels. These containers can collect broken glass for general disposal. However, general broken glass and needles potentially containing bloodborne pathogens should not be mixed and must be disposed of separately. The Occupational Safety and Health Administration requires that sharps containers be placed "Easily accessible to personnel and located as close as is feasible to the immediate area where sharps are used or can be reasonably anticipated to be found." (1910.1030(d)(4)(iii)(A)(2)(i) This ensures that the containers are kept close to where syringes, needles, and other (often medical) sharps devices are used. By doing this, proper and safe disposal practices are adhered to. The standard additionally references secondary containers, which are devices used to contain leakage or spillage. Oftentimes sharps placed in these containers still have small amounts of chemicals and medicines stored within them, posing a health and safety risk to workers if exposed. This leftover material is often known as "Dead Space".Disinfection/Decontamination
The decontamination process varies depending on the products and processes used. Many products, particularly sprays and aerosols, have a "contact time". The contact time is "...the time the product must be applied to the surface for it to be effective." This ensures that the chemicals has adequately removed or killed the contaminant. These chemicals are often designed to protect against HIV, Hepatitis B, and Hepatitis C. The EPA has a list of approved safe chemicals that can be used, each with different bloodborne pathogens they protect against. The efficacy of these chemicals is verified by OCSPP 810.2200.See also
*Infection control
Infection prevention and control (IPC) is the discipline concerned with preventing healthcare-associated infections; a practical rather than academic sub-discipline of epidemiology. In Northern Europe, infection prevention and control is expande ...
* Peggy Ferro
*Blood-borne disease
A blood-borne disease is a disease that can be spread through contamination by blood and other body fluids. Blood can contain pathogens of various types, chief among which are microorganisms, like bacteria and parasites, and non-living infectiou ...
*Sharps waste
Sharps waste is a form of biomedical waste composed of used "sharps", which includes any device or object used to puncture or lacerate the skin. Sharps waste is classified as biohazardous waste and must be carefully handled. Common medical mater ...
References
{{ReflistExternal links
W.H.O. Injection Safety Toolbox
W.H.O. Injection Safety
Centers for Disease Control – Injection Safety
• Washington Monthly, Jul/Aug 2010,
Medical equipment Drug delivery devices