SNAr Mechanism on:
[Wikipedia]
[Google]
[Amazon]
A nucleophilic aromatic substitution is a  This reaction differs from a common SN2 reaction, because it happens at a trigonal carbon atom (sp2 hybridization). The mechanism of SN2 reaction does not occur due to steric hindrance of the benzene ring. In order to attack the C atom, the nucleophile must approach in line with the C-LG (leaving group) bond from the back, where the benzene ring lies. It follows the general rule for which SN2 reactions occur only at a tetrahedral carbon atom.
The SN1 mechanism is possible but very unfavourable unless the leaving group is an exceptionally good one. It would involve the unaided loss of the leaving group and the formation of an aryl
This reaction differs from a common SN2 reaction, because it happens at a trigonal carbon atom (sp2 hybridization). The mechanism of SN2 reaction does not occur due to steric hindrance of the benzene ring. In order to attack the C atom, the nucleophile must approach in line with the C-LG (leaving group) bond from the back, where the benzene ring lies. It follows the general rule for which SN2 reactions occur only at a tetrahedral carbon atom.
The SN1 mechanism is possible but very unfavourable unless the leaving group is an exceptionally good one. It would involve the unaided loss of the leaving group and the formation of an aryl
 #The aromatic SN1 mechanism encountered with
#The aromatic SN1 mechanism encountered with  #The benzyne mechanism (E1cb-AdN)
#The benzyne mechanism (E1cb-AdN) #The
#The
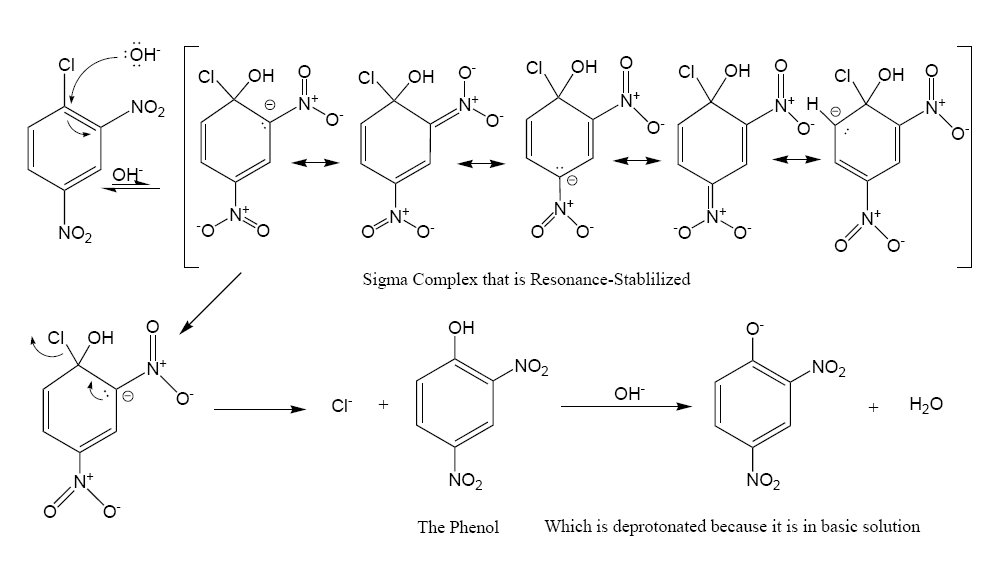 In this sequence the carbon atoms are numbered clockwise from 1–6 starting with the 1 carbon at 12 o'clock, which is bonded to the chlorine atom. Since the nitro group is an activator toward nucleophilic substitution, and a meta director, it allows the benzene carbon atom to which it is bonded to have a negative charge. In the Meisenheimer complex, the nonbonded electrons of the carbanion become bonded to the aromatic pi system which allows the ipso carbon atom to temporarily bond with the
In this sequence the carbon atoms are numbered clockwise from 1–6 starting with the 1 carbon at 12 o'clock, which is bonded to the chlorine atom. Since the nitro group is an activator toward nucleophilic substitution, and a meta director, it allows the benzene carbon atom to which it is bonded to have a negative charge. In the Meisenheimer complex, the nonbonded electrons of the carbanion become bonded to the aromatic pi system which allows the ipso carbon atom to temporarily bond with the


substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
in which the nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
displaces a good leaving group, such as a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a f ...
, on an aromatic ring
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satu ...
. Aromatic rings are usually nucleophilic, but some aromatic compounds do undergo nucleophilic substitution. Just as normally nucleophilic alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
can be made to undergo conjugate substitution if they carry electron-withdrawing substituents, so normally nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
aromatic rings also become electrophilic if they have the right substituents.cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
. In the SN1 reactions all the cations employed as intermediates were planar with an empty p orbital. This cation is planar but the p orbital is full (it is part of the aromatic ring) and the empty orbital is an sp2 orbital outside the ring.
The are six different mechanism by which aromatic rings undergo nucleophilic substitution.
Nucleophilic aromatic substitution mechanisms
There are 6nucleophilic substitution
In chemistry, a nucleophilic substitution is a class of chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass c ...
mechanisms encountered with aromatic systems:
#The SNAr (addition-elimination) mechanismdiazonium salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide.
General propert ...
sfree radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
SRN1 mechanism
# ANRORC mechanism
# Vicarious nucleophilic substitution.
The SNAr mechanism is the most important of these. Electron withdrawing groups activate the ring towards nucleophilic attack. For example if there are nitro functional groups positioned ortho or para
Para, or PARA, may refer to:
Businesses and organizations
* Paramount Global, traded as PARA on the Nasdaq stock exchange
* Para Group, the former name of CT Corp
* Para Rubber, now Skellerup, a New Zealand manufacturer
* Para USA, formerly ...
to the halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a f ...
leaving group, the SNAr mechanism is favored.
SNAr reaction mechanism
The following is thereaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage o ...
of a nucleophilic aromatic substitution of 2,4-dinitrochlorobenzene in a basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
solution in water.
 In this sequence the carbon atoms are numbered clockwise from 1–6 starting with the 1 carbon at 12 o'clock, which is bonded to the chlorine atom. Since the nitro group is an activator toward nucleophilic substitution, and a meta director, it allows the benzene carbon atom to which it is bonded to have a negative charge. In the Meisenheimer complex, the nonbonded electrons of the carbanion become bonded to the aromatic pi system which allows the ipso carbon atom to temporarily bond with the
In this sequence the carbon atoms are numbered clockwise from 1–6 starting with the 1 carbon at 12 o'clock, which is bonded to the chlorine atom. Since the nitro group is an activator toward nucleophilic substitution, and a meta director, it allows the benzene carbon atom to which it is bonded to have a negative charge. In the Meisenheimer complex, the nonbonded electrons of the carbanion become bonded to the aromatic pi system which allows the ipso carbon atom to temporarily bond with the hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
group (-OH). In order to return to a lower energy state, either the hydroxyl group leaves, or the chloride leaves. In solution both processes happen. A small percentage of the intermediate loses the chloride to become the product (2,4-dinitrophenol), while the rest return to the reactant. Since 2,4-dinitrophenol is in a lower energy state it will not return to form the reactant, so after some time has passed, the reaction reaches chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the ...
that favors the 2,4-dinitrophenol.
The formation of the resonance-stabilized Meisenheimer complex is slow because it is in a higher energy state than the aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
reactant. The loss of the chloride is fast, because the ring becomes aromatic again. Recent work indicates that, sometimes, the Meisenheimer complex is not always a true intermediate but may be the transition state of a 'frontside SN2' process, particularly if stabilization by electron-withdrawing groups is not very strong. A 2019 review argues that such 'concerted SNAr' reactions are more prevalent than previously assumed.
Aryl halides cannot undergo the classic 'backside' SN2 reaction. The carbon-halogen bond is in the plane of the ring because the carbon atom has a trigonal planar geometry. Backside attack is blocked and this reaction is therefore not possible. An SN1 reaction is possible but very unfavourable. It would involve the unaided loss of the leaving group and the formation of an aryl cation. The nitro group is the most commonly encountered activating group, other groups are the cyano and the acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group ( I ...
group. The leaving group can be a halogen or a sulfide. With increasing electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
the reaction rate for nucleophilic attack increases. This is because the rate-determining step for an SNAr reaction is attack of the nucleophile and the subsequent breaking of the aromatic system; the faster process is the favourable reforming of the aromatic system after loss of the leaving group. As such, the following pattern is seen with regard to halogen leaving group ability for SNAr: F > Cl ≈ Br > I (i.e. an inverted order to that expected for an SN2 reaction). If looked at from the point of view of an SN2 reaction this would seem counterintuitive, since the C-F bond is amongst the strongest in organic chemistry, when indeed the fluoride is the ideal leaving group for an SNAr due to the extreme polarity of the C-F bond. Nucleophiles can be amines, alkoxides, sulfides and stabilized carbanions
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
.
Nucleophilic aromatic substitution reactions
Some typical substitution reactions on arenes are listed below. * In the Bamberger rearrangement N-phenylhydroxylamines rearrange to 4-aminophenols. The nucleophile is water. * In the Sandmeyer reaction diazonium salts react with halides. * The Smiles rearrangement is the intramolecular version of this reaction type. Nucleophilic aromatic substitution is not limited to arenes, however; the reaction takes place even more readily withheteroarene
Simple aromatic rings, also known as simple arenes or simple aromatics, are aromatic organic compounds that consist only of a conjugated planar ring system. Many simple aromatic rings have trivial names. They are usually found as substructures of ...
s. Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakl ...
s are especially reactive when substituted in the aromatic ortho position
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.
''Ortho'', ''meta'', and ''para'' substitution
* I ...
or aromatic para position because then the negative charge is effectively delocalized at the nitrogen position. One classic reaction is the Chichibabin reaction ( Aleksei Chichibabin, 1914) in which pyridine is reacted with an alkali-metal amide such as sodium amide to form 2-aminopyridine.
In the compound methyl 3-nitropyridine-4-carboxylate, the ''meta'' nitro group is actually displaced by fluorine with cesium fluoride in DMSO at 120 °C.

Asymmetric nucleophilic aromatic substitution
With carbon nucleophiles such as 1,3-dicarbonyl compounds the reaction has been demonstrated as a method for theasymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecu ...
of chiral molecules. First reported in 2005, the organocatalyst (in a dual role with that of a phase transfer catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of heterogeneous catalysis. Ionic ...
) is derived from cinchonidine (benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a subst ...
ated at N and O).
:
See also
*Electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
* Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
* Substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
* SN1 reaction
* SN2 reaction
* SNi reaction
* Nucleophilic aliphatic substitution
* Nucleophilic acyl substitution
References
{{Reaction mechanisms Nucleophilic substitution reactions Reaction mechanisms