SF40 Full Opencomp on:
[Wikipedia]
[Google]
[Amazon]
Sulfur tetrafluoride is a
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the formula S F4. It is a colorless corrosive gas that releases dangerous hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
gas upon exposure to water or moisture. Sulfur tetrafluoride is a useful reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries.
Structure
Sulfur in SF4 is in the +4oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
, with one lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electrons. The atoms in SF4 are arranged in a see-saw shape, with the sulfur atom at the center. One of the three equatorial positions is occupied by a nonbonding lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded Octet rule, octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. P ...
molecules to be bonded less strongly.
The 19F NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
spectrum of SF4 reveals only one signal, which indicates that the axial and equatorial F atom positions rapidly interconvert via pseudorotation
In chemistry, a pseudorotation is a set of intramolecular movements of attached groups (i.e., ligands) on a highly symmetric molecule, leading to a molecule indistinguishable from the initial one. The International Union of Pure and Applied Chem ...
.
Synthesis and manufacture
At the laboratory scale, sulfur tetrafluoride is prepared from elemental sulfur andcobaltic fluoride
Cobalt(III) fluoride is the inorganic compound with the formula . Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It is used to synthesize organofluorine compounds.
The related cobalt(III) chloride is also known but ...
:S + 4CoF3 → SF4 + 4CoF2
SF4 is industrially produced by the reaction of SCl2 and NaF with acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
as a catalyst
:3 SCl2 + 4 NaF → SF4 + S2Cl2 + 4 NaCl
At higher temperatures (e.g. 225–450 °C), the solvent is superfluous. Moreover, sulfur dichloride may be replaced by elemental sulfur (S) and chlorine (Cl2).
A low-temperature (e.g. 20–86 °C) alternative to the chlorinative process above uses liquid bromine (Br2) as oxidant and solvent:
:S(s) + 2 Br2(l; excess) + 4KF(s) → SF4↑ + 4 KBr(brom)
Use in synthesis of organofluorine compounds
Inorganic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
, SF4 is used to convert COH and C=O groups into CF and CF2 groups, respectively. The efficiency of these conversions are highly variable.
In the laboratory, the use of SF4 has been superseded by the safer and more easily handled diethylaminosulfur trifluoride, (C2H5)2NSF3, "DAST": This reagent is prepared from SF4:
:
Other reactions
Sulfur chloride pentafluoride (), a useful source of the SF5 group, is prepared from SF4. : Hydrolysis of SF4 givessulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
:
:SF4 + 2 H2O → SO2 + 4 HF
This reaction proceeds via the intermediacy of thionyl fluoride
Thionyl fluoride is the inorganic compound with the formula . This colourless gas is mainly of theoretical interest, but it is a product of the degradation of sulfur hexafluoride, an insulator in electrical equipment. The molecule adopts a distort ...
, which usually does not interfere with the use of SF4 as a reagent.
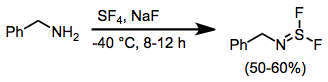
When amines are treated with SF4 and a base, aminosulfur difluorides result.

Toxicity
reacts inside the lungs with moisture, formingsulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a colorless gas with a pungent smell that is responsible for the odor of burnt matches. It is r ...
and hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
which forms highly toxic and corrosive hydrofluoric acid
References
{{Sulfur compounds Sulfur fluorides Fluorinating agents Hypervalent molecules ja:フッ化硫黄#四フッ化硫黄