relativistic quantum chemistry on:
[Wikipedia]
[Google]
[Amazon]
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate
 One of the most important and familiar results of relativity is that the
One of the most important and familiar results of relativity is that the  It follows that
:
At right, the above ratio of the relativistic and nonrelativistic Bohr radii has been plotted as a function of the electron velocity. Notice how the relativistic model shows the radius decreases with increasing velocity.
When the Bohr treatment is extended to hydrogenic atoms, the Bohr radius becomes
:
where is the
It follows that
:
At right, the above ratio of the relativistic and nonrelativistic Bohr radii has been plotted as a function of the electron velocity. Notice how the relativistic model shows the radius decreases with increasing velocity.
When the Bohr treatment is extended to hydrogenic atoms, the Bohr radius becomes
:
where is the
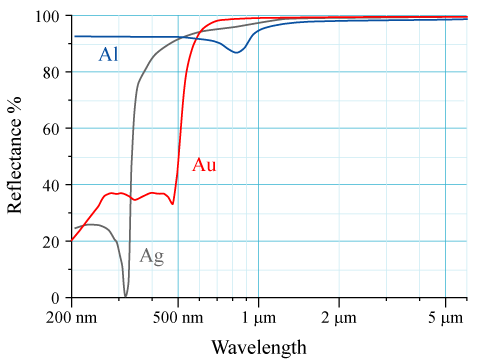 The reflectivity of
The reflectivity of
elemental
An elemental is a mythic being that is described in occult and alchemical works from around the time of the European Renaissance, and particularly elaborated in the 16th century works of Paracelsus. According to Paracelsus and his subsequent fo ...
properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile me ...
: due to relativistic effects, it is not silvery like most other metals.
The term ''relativistic effects'' were developed in light of the history of quantum mechanics. Initially, quantum mechanics was developed without considering the theory of relativity
The theory of relativity usually encompasses two interrelated theories by Albert Einstein: special relativity and general relativity, proposed and published in 1905 and 1915, respectively. Special relativity applies to all physical phenomena in ...
. Relativistic effects are those discrepancies between values calculated by models that consider relativity and those that do not. Relativistic effects are important for heavier elements with high atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
s, such as lanthanides and actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s.
Relativistic effects in chemistry can be considered to be perturbations, or small corrections, to the non-relativistic theory of chemistry, which is developed from the solutions of the Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
. These corrections affect the electrons differently depending on the electron speed compared with the speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant that is important in many areas of physics. The speed of light is exactly equal to ). According to the special theory of relativity, is the upper limit ...
. Relativistic effects are more prominent in heavy elements because only in these elements do electrons attain sufficient speeds for the elements to have properties that differ from what non-relativistic chemistry predicts.
History
Beginning in 1935,Bertha Swirles
Bertha Swirles, Lady Jeffreys (22 May 1903 – 18 December 1999) was an English physicist, academic and scientific author who carried out research on quantum theory in its early days. She was associated with Girton College, University of Cam ...
described a relativistic treatment of a many-electron system, despite Paul Dirac
Paul Adrien Maurice Dirac (; 8 August 1902 – 20 October 1984) was an English theoretical physicist who is regarded as one of the most significant physicists of the 20th century. He was the Lucasian Professor of Mathematics at the Univer ...
's 1929 assertion that the only imperfections remaining in quantum mechanics "give rise to difficulties only when high-speed particles are involved and are therefore of no importance in the consideration of the atomic and molecular structure and ordinary chemical reactions in which it is, indeed, usually sufficiently accurate if one neglects relativity variation of mass and velocity and assumes only Coulomb forces between the various electrons and atomic nuclei".
Theoretical chemists by and large agreed with Dirac's sentiment until the 1970s, when relativistic effects were observed in heavy elements. The Schrödinger equation
The Schrödinger equation is a linear partial differential equation that governs the wave function of a quantum-mechanical system. It is a key result in quantum mechanics, and its discovery was a significant landmark in the development of th ...
had been developed without considering relativity in Schrödinger's 1926 article. Relativistic corrections were made to the Schrödinger equation (see Klein–Gordon equation
The Klein–Gordon equation (Klein–Fock–Gordon equation or sometimes Klein–Gordon–Fock equation) is a relativistic wave equation, related to the Schrödinger equation. It is second-order in space and time and manifestly Lorentz-covariant ...
) to describe the fine structure of atomic spectra, but this development and others did not immediately trickle into the chemical community. Since atomic spectral line
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter w ...
s were largely in the realm of physics and not in that of chemistry, most chemists were unfamiliar with relativistic quantum mechanics, and their attention was on lighter elements typical for the organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, ...
focus of the time.
Dirac's opinion on the role relativistic quantum mechanics would play for chemical systems is wrong for two reasons. First, electrons in ''s'' and ''p'' atomic orbitals
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any sp ...
travel at a significant fraction of the speed of light. Second, relativistic effects give rise to indirect consequences that are especially evident for ''d'' and ''f'' atomic orbitals.
Qualitative treatment
relativistic mass
The word "mass" has two meanings in special relativity: '' invariant mass'' (also called rest mass) is an invariant quantity which is the same for all observers in all reference frames, while the relativistic mass is dependent on the velocity o ...
of the electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no ...
increases as
:
where are the electron rest mass
The electron mass (symbol: ''m''e) is the mass of a stationary electron, also known as the invariant mass of the electron. It is one of the fundamental constants of physics. It has a value of about or about , which has an energy-equivalent of a ...
, velocity
Velocity is the directional speed of an object in motion as an indication of its rate of change in position as observed from a particular frame of reference and as measured by a particular standard of time (e.g. northbound). Velocity i ...
of the electron, and speed of light
The speed of light in vacuum, commonly denoted , is a universal physical constant that is important in many areas of physics. The speed of light is exactly equal to ). According to the special theory of relativity, is the upper limit ...
respectively. The figure at the right illustrates this relativistic effect as a function of velocity.
This has an immediate implication on the Bohr radius
The Bohr radius (''a''0) is a physical constant, approximately equal to the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an ...
(), which is given by
:
where is the reduced Planck's constant
The Planck constant, or Planck's constant, is a fundamental physical constant of foundational importance in quantum mechanics. The constant gives the relationship between the energy of a photon and its frequency, and by the mass-energy equivalen ...
, and α is the fine-structure constant
In physics, the fine-structure constant, also known as the Sommerfeld constant, commonly denoted by (the Greek letter ''alpha''), is a fundamental physical constant which quantifies the strength of the electromagnetic interaction between el ...
(a relativistic correction for the Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar Syst ...
).
Arnold Sommerfeld calculated that, for a 1s orbital electron of a hydrogen atom with an orbiting radius of 0.0529 nm, α ≈ 1/137. That is to say, the fine-structure constant
In physics, the fine-structure constant, also known as the Sommerfeld constant, commonly denoted by (the Greek letter ''alpha''), is a fundamental physical constant which quantifies the strength of the electromagnetic interaction between el ...
shows the electron traveling at nearly 1/137 the speed of light. One can extend this to a larger element with an atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
''Z'' by using the expression for a 1s electron, where ''v'' is its radial velocity, i.e., its instantaneous speed tangent to the radius of the atom. For gold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile me ...
with ''Z'' = 79, ''v'' ≈ 0.58''c'', so the 1s electron will be moving at 58% of the speed of light. Plugging this in for ''v''/''c'' in the equation for the relativistic mass, one finds that ''m''rel = 1.22''m''e, and in turn putting this in for the Bohr radius above one finds that the radius shrinks by 22%.
If one substitutes the "relativistic mass" into the equation for the Bohr radius it can be written
:
 It follows that
:
At right, the above ratio of the relativistic and nonrelativistic Bohr radii has been plotted as a function of the electron velocity. Notice how the relativistic model shows the radius decreases with increasing velocity.
When the Bohr treatment is extended to hydrogenic atoms, the Bohr radius becomes
:
where is the
It follows that
:
At right, the above ratio of the relativistic and nonrelativistic Bohr radii has been plotted as a function of the electron velocity. Notice how the relativistic model shows the radius decreases with increasing velocity.
When the Bohr treatment is extended to hydrogenic atoms, the Bohr radius becomes
:
where is the principal quantum number
In quantum mechanics, the principal quantum number (symbolized ''n'') is one of four quantum numbers assigned to each electron in an atom to describe that electron's state. Its values are natural numbers (from 1) making it a discrete variable.
A ...
, and ''Z'' is an integer for the atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
. In the Bohr model
In atomic physics, the Bohr model or Rutherford–Bohr model, presented by Niels Bohr and Ernest Rutherford in 1913, is a system consisting of a small, dense nucleus surrounded by orbiting electrons—similar to the structure of the Solar Syst ...
, the angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed syst ...
is given as . Substituting into the equation above and solving for gives
:
:
:
From this point, atomic units
The Hartree atomic units are a system of natural units of measurement which is especially convenient for atomic physics and computational chemistry calculations. They are named after the physicist Douglas Hartree. By definition, the following four ...
can be used to simplify the expression into
:
Substituting this into the expression for the Bohr ratio mentioned above gives
:
At this point one can see that a low value of and a high value of results in . This fits with intuition: electrons with lower principal quantum numbers will have a higher probability density of being nearer to the nucleus. A nucleus with a large charge will cause an electron to have a high velocity. A higher electron velocity means an increased electron relativistic mass, and as a result the electrons will be near the nucleus more of the time and thereby contract the radius for small principal quantum numbers.
Periodic-table deviations
The periodic table was constructed by scientists who noticed periodic trends in known elements of the time. Indeed, the patterns found in it are what give the periodic table its power. Many of the chemical and physical differences between the 5th period ( Rb– Xe) and the 6th period ( Cs– Rn) arise from the larger relativistic effects of the latter. These relativistic effects are particularly large forgold
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile me ...
and its neighbors platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
and mercury. An important quantum relativistic effect is the van der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
.
Mercury
Mercury (Hg) is a liquid down to −39 °C (see theMelting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
). Bonding forces are weaker for Hg–Hg bonds than for their immediate neighbors such as cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, silvery-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of ...
(m.p. 321 °C) and gold (m.p. 1064 °C). The lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii ...
only partially accounts for this anomaly. Mercury in the gas phase is alone among metals in that it is quite typically found in a monomeric form as Hg(g). Hg22+(g) also forms, and it is a stable species due to the relativistic shortening of the bond.
Hg2(g) does not form because the 6s2 orbital is contracted by relativistic effects and may therefore only weakly contribute to any bonding; in fact, Hg–Hg bonding must be mostly the result of van der Waals forces
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
, which explains why the bonding for Hg–Hg is weak enough to allow for Hg to be a liquid at room temperature.
Au2(g) and Hg(g) are analogous with H2(g) and He(g) with regard to having the same nature of difference. The relativistic contraction of the 6s2 orbital leads to gaseous mercury sometimes being referred to as a pseudo noble gas
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low ch ...
.
Color of gold and caesium
 The reflectivity of
The reflectivity of aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. I ...
(Al), silver (Ag), and gold (Au) is shown in the graph to the right. The human eye sees electromagnetic radiation with a wavelength near 600 nm as yellow. Gold appears yellow because it absorbs blue light more than it absorbs other visible wavelengths of light; the reflected light reaching the eye is therefore lacking in blue compared with the incident light. Since yellow is complementary to blue, this makes a piece of gold under white light appear yellow to human eyes.
The electronic transition from the 5d orbital to the 6s orbital is responsible for this absorption. An analogous transition occurs in silver, but the relativistic effects are smaller than in gold. While silver's 4d orbital experiences some relativistic expansion and the 5s orbital contraction, the 4d–5s distance in silver is much greater than the 5d–6s distance in gold. The relativistic effects increase the 5d orbital's distance from the atom's nucleus and decrease the 6s orbital's distance.
Caesium, the heaviest of the alkali metals that can be collected in quantities sufficient for viewing, has a golden hue, whereas the other alkali metals are silver-white. However, relativistic effects are not very significant at ''Z'' = 55 for caesium (not far from ''Z'' = 47 for silver). The golden color of caesium comes from the decreasing frequency of light required to excite electrons of the alkali metals as the group is descended. For lithium through rubidium, this frequency is in the ultraviolet, but for caesium it reaches the blue-violet end of the visible spectrum; in other words, the plasmonic frequency of the alkali metals becomes lower from lithium to caesium. Thus caesium transmits and partially absorbs violet light preferentially, while other colors (having lower frequency) are reflected; hence it appears yellowish.
Lead–acid battery
Without relativity,lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
(''Z'' = 82) would be expected to behave much like tin (''Z'' = 50), so tin–acid batteries should work just as well as the lead–acid batteries commonly used in cars. However, calculations show that about 10 V of the 12 V produced by a 6-cell lead–acid battery arises purely from relativistic effects, explaining why tin–acid batteries do not work.
Inert-pair effect
In Tl(I) (thallium
Thallium is a chemical element with the symbol Tl and atomic number 81. It is a gray post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Chemists William Crookes an ...
), Pb(II) (lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cu ...
), and Bi(III) (bismuth
Bismuth is a chemical element with the symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs ...
) complexes a 6s2 electron pair exists. The inert pair effect is the tendency of this pair of electrons to resist oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
due to a relativistic contraction of the 6s orbital.
Other effects
Additional phenomena commonly caused by relativistic effects are the following: * The effect of relativistic effects onmetallophilic interaction
In chemistry, a metallophilic interaction is defined as a type of non-covalent attraction between heavy metal atoms. The atoms are often within Van der Waals distance of each other and are about as strong as hydrogen bonds. The effect can be in ...
s is uncertain. Although Runeberg ''et al.'' (1999) calculated an attractive effect, Wan ''et al.'' (2021) instead calculated a repulsive effect.
* The stability of gold and platinum anions in compounds such as caesium auride.
* The slightly reduced reactivity of francium compared with caesium.
* About 10% of the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii ...
is attributed to the relativistic mass of high-velocity electrons and the smaller Bohr radius
The Bohr radius (''a''0) is a physical constant, approximately equal to the most probable distance between the nucleus and the electron in a hydrogen atom in its ground state. It is named after Niels Bohr, due to its role in the Bohr model of an ...
that results.
See also
* Ionization energy *Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
*Electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
Note that this is ...
*Quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistr ...
References
Further reading
*P. A. Christiansen; W. C. Ermler; K. S. Pitzer. Relativistic Effects in Chemical Systems. ''Annual Review of Physical Chemistry'' 1985, ''36'', 407–432. {{doi, 10.1146/annurev.pc.36.100185.002203 Quantum chemistry Special relativity