Retrosynthetic analysis on:
[Wikipedia]
[Google]
[Amazon]
Retrosynthetic analysis is a technique for solving problems in the planning of
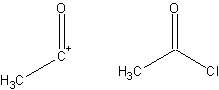 ;Target: The desired final compound.
;Transform: The reverse of a synthetic reaction; the formation of starting materials from a single product.
;Target: The desired final compound.
;Transform: The reverse of a synthetic reaction; the formation of starting materials from a single product.
 In planning the synthesis, two synthons are identified. A nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Both synthons do not exist as written; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the −COOH synthon, while benzyl bromide is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
: PhCH2Br + NaCN → PhCH2CN + NaBr
: PhCH2CN + 2 H2O → PhCH2COOH + NH3
In planning the synthesis, two synthons are identified. A nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Both synthons do not exist as written; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the −COOH synthon, while benzyl bromide is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
: PhCH2Br + NaCN → PhCH2CN + NaBr
: PhCH2CN + 2 H2O → PhCH2COOH + NH3
 In fact, phenylacetic acid has been synthesized from benzyl cyanide, itself prepared by the analogous reaction of benzyl bromide with
In fact, phenylacetic acid has been synthesized from benzyl cyanide, itself prepared by the analogous reaction of benzyl bromide with
ChemAIRS, AI-driven retrosynthesis tools by Chemical.AI
Centre for Molecular and Biomolecular Informatics
Presentation on ARChem Route Designer, ACS, Philadelphia, September 2008
for more info on ARChem see the SimBioSys pages.
Manifold, Software freely available for academic users developed by PostEra
Retrosynthesis planning tool: ICSynth by InfoChem
Spaya, Software freely available proposed by Iktos
{{Branches of chemistry Chemical synthesis Organic chemistry Chemical reaction engineering
organic syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and expe ...
. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. Each precursor material is examined using the same method. This procedure is repeated until simple or commercially available structures are reached. These simpler/commercially available compounds can be used to form a synthesis of the target molecule. Retrosynthetic analysis was used as early as 1917
Events
Below, the events of World War I have the "WWI" prefix.
January
* January 9 – WWI – Battle of Rafa: The last substantial Ottoman Army garrison on the Sinai Peninsula is captured by the Egyptian Expeditionary Force's ...
in Robinson's Tropinone total synthesis. Important conceptual work on retrosynthetic analysis was published by George Vladutz in 1963
Events January
* January 1 – Bogle–Chandler case: Commonwealth Scientific and Industrial Research Organisation scientist Dr. Gilbert Bogle and Mrs. Margaret Chandler are found dead (presumed poisoned), in bushland near the Lane Cove ...
.
E.J. Corey formalized and popularized the concept from 1967 onwards in his article ''General methods for the construction of complex molecules'' and his book ''The Logic of Chemical Synthesis''.
The power of retrosynthetic analysis becomes evident in the design of a synthesis. The goal of retrosynthetic analysis is a structural simplification. Often, a synthesis will have more than one possible synthetic route. Retrosynthesis is well suited for discovering different synthetic routes and comparing them in a logical and straightforward fashion. A database may be consulted at each stage of the analysis, to determine whether a component already exists in the literature. In that case, no further exploration of that compound would be required. If that compound exists, it can be a jumping point for further steps developed to reach a synthesis.
There are both academic and commercial groups developing retrosynthesis tools. With the growing application of machine learning and artificial intelligence in chemistry, many research groups, such as the Coley Group from MIT, and companies, such as Chemical.AI, Reaxys, etc., have started to integrate deep learning into the conventional rule-based approaches.
Definitions
;Disconnection: A retrosynthetic step involving the breaking of a bond to form two (or more) synthons. ;Retron: A minimal molecular substructure that enables certain transformations. ;Retrosynthetic tree: Adirected acyclic graph
In mathematics, particularly graph theory, and computer science, a directed acyclic graph (DAG) is a directed graph with no directed cycles. That is, it consists of vertices and edges (also called ''arcs''), with each edge directed from one ...
of several (or all) possible retrosyntheses of a single target.
;Synthon: A fragment of a compound that assists in the formation of a synthesis, derived from that target molecule. A synthon and the corresponding commercially available synthetic equivalent are shown below:
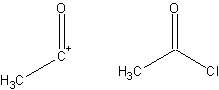 ;Target: The desired final compound.
;Transform: The reverse of a synthetic reaction; the formation of starting materials from a single product.
;Target: The desired final compound.
;Transform: The reverse of a synthetic reaction; the formation of starting materials from a single product.
Example
Shown below is a retrosynthetic analysis of phenylacetic acid:sodium cyanide
Sodium cyanide is a compound with the formula Na C N and the structure . It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also expl ...
.
Strategies
Functional group strategies
Manipulation offunctional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s can lead to significant reductions in molecular complexity.
Stereochemical strategies
Numerous chemical targets have distinct stereochemical demands. Stereochemical transformations (such as the Claisen rearrangement and Mitsunobu reaction) can remove or transfer the desired chirality thus simplifying the target.Structure-goal strategies
Directing a synthesis toward a desirable intermediate can greatly narrow the focus of analysis. This allows bidirectional search techniques.Transform-based strategies
The application of transformations to retrosynthetic analysis can lead to powerful reductions in molecular complexity. Unfortunately, powerful transform-based retrons are rarely present in complex molecules, and additional synthetic steps are often needed to establish their presence.Topological strategies
The identification of one or more key bond disconnections may lead to the identification of key substructures or difficult to identify rearrangement transformations in order to identify the key structures. * Disconnections that preserve ring structures are encouraged. * Disconnections that create rings larger than 7 members are discouraged. *Disconnection involves creativity.See also
*Organic synthesis
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the gen ...
* Total synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
References
External links
ChemAIRS, AI-driven retrosynthesis tools by Chemical.AI
Centre for Molecular and Biomolecular Informatics
Presentation on ARChem Route Designer, ACS, Philadelphia, September 2008
for more info on ARChem see the SimBioSys pages.
Manifold, Software freely available for academic users developed by PostEra
Retrosynthesis planning tool: ICSynth by InfoChem
Spaya, Software freely available proposed by Iktos
{{Branches of chemistry Chemical synthesis Organic chemistry Chemical reaction engineering