Reaction intermediate on:
[Wikipedia]
[Google]
[Amazon]
In
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, a reaction intermediate, or intermediate, is a molecular entity
In chemistry and physics, a molecular entity, or chemical entity, is "any constitutionally or isotopically distinct atom, molecule, ion, ion pair, radical, radical ion, complex, conformer, etc., identifiable as a separately distinguishable en ...
arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding intermediates, but is consumed in a later step. It does not appear in the chemical equation for the overall reaction.
For example, consider this hypothetical reaction:
:A + B → C + D
If this overall reaction comprises two elementary steps thus:
:A + B → X
:X → C + D
then X is a reaction intermediate.
The phrase ''reaction intermediate'' is often abbreviated to the single word ''intermediate'', and this is IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
's preferred form of the term. But this shorter form has other uses. It often refers to reactive intermediates. It is also used more widely for chemicals such as cumene which are traded within the chemical industry but are not generally of value outside it.
IUPAC definition
TheIUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
Gold Book defines an ''intermediate'' as a compound that has a lifetime greater than a molecular vibration, is formed (directly or indirectly) from the reactants, and reacts further to give (either directly or indirectly) the products of a chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
. The lifetime condition distinguishes true, chemically distinct intermediates, both from vibrational states and from transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
s (which, by definition, have lifetimes close to that of molecular vibration).
The different steps of a multi-step reaction often differ widely in their reaction rates. Where the difference is significant, an intermediate consumed more quickly than another may be described as a ''relative'' intermediate. A reactive intermediate is one which due to its short lifetime does not remain in the product mixture. Reactive intermediates are usually high-energy, are unstable and are seldom isolated.
Common reaction intermediates
Carbocations
Cations, often carbocations, serve as intermediates in various types of reactions to synthesize new compounds.Carbocation intermediates in alkene addition
Carbocations are formed in two major alkene addition reactions. In an HX addition reaction, thepi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
of an alkene acts as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
and bonds with the proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
of an HX molecule, where the X is a halogen atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
. This forms a carbocation intermediate, and the X then bonds to the positive carbon that is available, as in the following two-step reaction.
:
:
Similarly, in an addition reaction, the pi bond of an alkene acts as a nucleophile and bonds with the proton of an molecule. This forms a carbocation intermediate (and an atom); the oxygen atom of then bonds with the positive carbon of the intermediate. The oxygen finally deprotonates to form a final alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
product, as follows.
:
:
:
Carbocation intermediates in nucleophilic substitution
Nucleophilic substitution reactions occur when a nucleophilic molecule attacks a positive or partially positive electrophilic center by breaking and creating a new bond. SN1 and SN2 are two different mechanisms for nucleophilic substitution, and SN1 involves a carbocation intermediate. In SN1, a leaving group is broken off to create a carbocation reaction intermediate. Then, a nucleophile attacks and forms a new bond with the carbocation intermediate to form the final, substituted product, as shown in the reaction of 2-bromo-2-methylpropane to form 2-methyl-2-propanol. : : : In this reaction, is the formed carbocation intermediate to form the alcohol product.Carbocation intermediates in elimination reactions
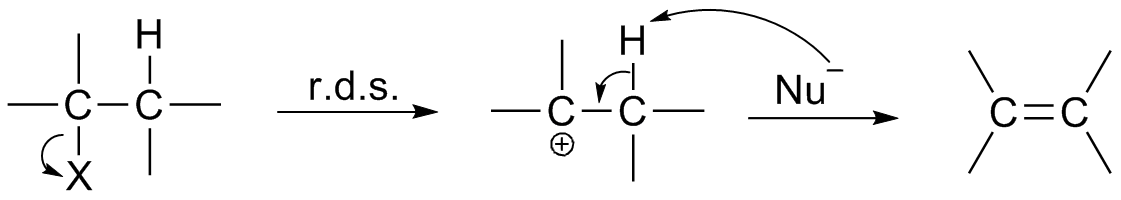
β-elimination or elimination reactions occur through the loss of a substituent leaving group and loss of a proton to form a pi bond. E1 and E2 are two different mechanisms for elimination reactions, and E1 involves a carbocation intermediate. In E1, a leaving group detaches from a carbon to form a carbocation reaction intermediate. Then, a solvent removes a proton, but theelectron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s used to form the proton bond form a pi bond, as shown in the pictured reaction on the right.

Carboanions
A carboanion is an organic molecule where a carbon atom is not electron deficient but contain an overall negative charge. Carboanions are strong nucleophiles, which can be used to extend an alkene's carbon backbone in the synthesis reaction shown below. : : The alkyne carbanion, , is a reaction intermediate in this reaction.Radicals
Radicals are highly reactive and short-lived, as they have an unpaired electron which makes them extremely unstable. Radicals often react with hydrogens attached to carbon molecules, effectively making the carbon a radical while stabilizing the former radical in a process called propagation. The formed product, a carbon radical, can react with non-radical molecule to continue propagation or react with another radical to form a new stable molecule such as a longer carbon chain or an alkyl halide. The example below of methane chlorination shows a multi-step reaction involving radicals.Methane chlorination
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
chlorination is a chain reaction. If only the products and reactants are analyzed, the result is:
:
However, this reaction has 3 intermediate reactants which are formed during a sequence of 4 irreversible second order reactions until we arrive at the final product. This is why it is called a chain reaction. Following only the carbon containing species in series:
:
Reactants:
Products:
The other species are reaction intermediates:
These are the set of irreversible second-order reactions:
:
:
:
:
These intermediate species' concentrations can be calculated by integrating the system of kinetic equations. The full reaction is a free radical propagation reaction which is filled out in detail below.
Initiation: This reaction can occur by thermolysis (heating) or photolysis (absorption of light) leading to the breakage of a molecular chlorine bond.
:
When the bond is broken it produces two highly reactive chlorine atoms.
Propagation: This stage has two distinct reaction classes. The first is the stripping of a hydrogen from the carbon species by the chlorine radicals. This occurs because chlorine atoms alone are unstable, and these chlorine atoms react with one the carbon species' hydrogens. The result is the formation of hydrochloric acid and a new radical methyl group.
:
:
:
:
These new radical carbon containing species now react with a second molecule. This regenerates the chlorine radical and the cycle continues. This reaction occurs because while the radical methyl species are more stable than the radical chlorines, the overall stability of the newly formed chloromethane species more than makes up the energy difference.
:
:
:
:
During the propagation of the reaction, there are several highly reactive species that will be removed and stabilized at the termination step.
Termination: This kind of reaction takes place when the radical species interact directly. The products of the termination reactions are typically very low yield in comparison to the main products or intermediates as the highly reactive radical species are in relatively low concentration in relation to the rest of the mixture. This kind of reaction produces stable side products, reactants, or intermediates and slows the propagation reaction by lowering the number of radicals available to propagate the chain reaction.
There are many different termination combinations, some examples are:
Union of methyl radicals from a C-C bond leading to ethane (a side product).
:
Union of one methyl radical to a Cl radical forming chloromethane (another reaction forming an intermediate).
:
Union of two Cl radicals to reform chlorine gas (a reaction reforming a reactant).
:
Applications
Biological intermediates
Reaction intermediates serve purposes in a variety of biological settings. An example of this is demonstrated with the enzyme reaction intermediate of metallo-β-lactamase, whichbacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
can use to acquire resistance to commonly used antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
s such as penicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
. Metallo-β-lactamase can catalyze β-lactams, a family of common antibiotics. Spectroscopy techniques have found that the reaction intermediate of metallo-β-lactamase uses zinc in the resistance pathway.
Another example of the importance of reaction intermediates is seen with AAA-ATPase p97, a protein that used in a variety of cellular metabolic processes. p97 is also linked to degenerative disease and cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
. In a study looking at reaction intermediates of the AAA-ATPase p97 function found an important ADP.Pi nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
intermediate is important in the p97 molecular operation.
An additional example of biologically relevant reaction intermediates can be found with the RCL enzymes, which catalyzes glycosidic bonds. When studied using methanolysis, it was found that the reaction required the formation of a reaction intermediate.
Chemical processing industry
In the chemical industry, the term ''intermediate'' may also refer to the (stable) product of a reaction that is itself valuable only as a precursor chemical for other industries. A common example is cumene which is made frombenzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
and propylene and used to make acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
and phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
in the cumene process. The cumene itself is of relatively little value in and of itself, and is typically only bought and sold by chemical companies.
See also
* Activated complexReferences
{{Authority control Chemical kinetics Chemical reactions