Radical-nucleophilic Aromatic Substitution on:
[Wikipedia]
[Google]
[Amazon]
Radical-nucleophilic aromatic substitution or SRN1 in  The substituent X is a
The substituent X is a
 In this
In this  The
The 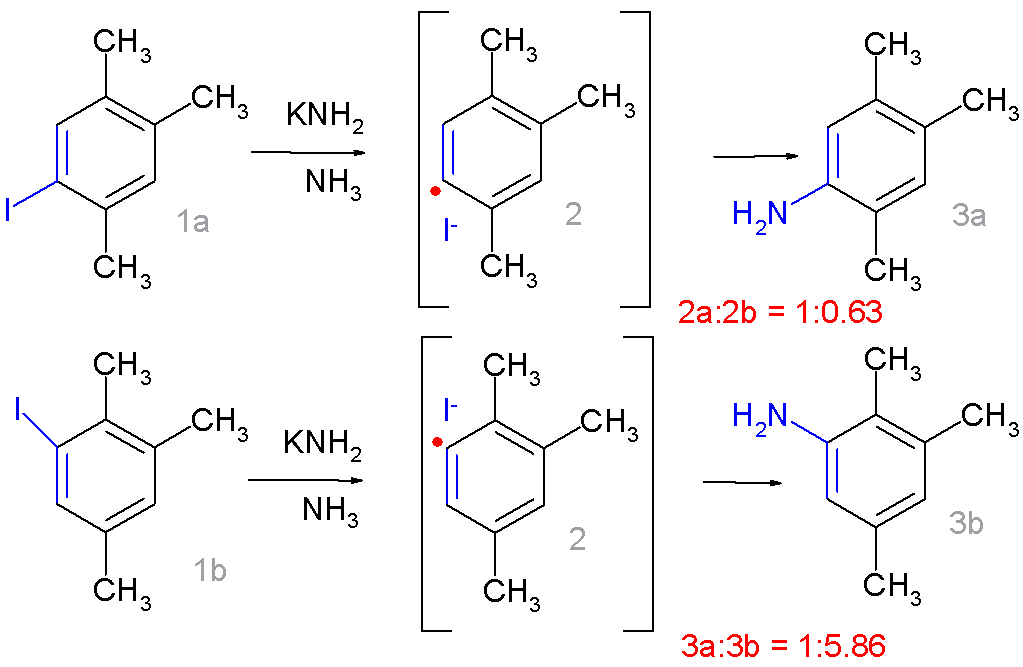 It now resembles ''ipso''-substitution with 1a forming preferentially 3a and 1b forming 3b.
It now resembles ''ipso''-substitution with 1a forming preferentially 3a and 1b forming 3b.
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
is a type of substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
in which a certain substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
on an aromatic compound
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
is replaced by a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
through an intermediary free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
species:
 The substituent X is a
The substituent X is a halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
and nucleophiles can be sodium amide
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the inorganic compound with the formula . It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is whit ...
, an alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organyl substituent. Alkoxides are strong bases and, whe ...
or a carbon nucleophile such as an enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the Organic synthesis, synthesis of organic compounds.
Bonding and structure
Enolate ...
. In contrast to regular nucleophilic aromatic substitution
A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic c ...
, deactivating group
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing group, directing effect on positional isomer of the product (chemistry), products that are fo ...
s on the arene are not required.
This reaction type was discovered in 1970 by Bunnett and Kim and the abbreviation SRN1 stands for substitution radical-nucleophilic unimolecular as it shares properties with an aliphatic SN1 reaction. An example of this reaction type is the Sandmeyer reaction
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts using copper salts as reagents or catalysts.
It is an example of a radical-nucleophilic aromatic substitution. The Sandmeyer reaction provides ...
.
Reaction mechanism
 In this
In this radical substitution
In organic chemistry, a radical-substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.March Jerry; (1985). Advanced organic chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wil ...
the aryl halide 1 accepts an electron from a radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical in ...
forming a radical anion
In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a ...
2. This intermediate collapses into an aryl radical An aryl radical in organic chemistry is a reactive intermediate and an arene compound incorporating one free radical carbon atom as part of the ring structure. As such it is the radical counterpart of the arenium ion. The parent compound is the ...
3 and a halide anion. The aryl radical reacts with the nucleophile 4 to a new radical anion 5 which goes on to form the substituted product by transferring its electron to new aryl halide in the chain propagation
In chemistry, chain propagation (sometimes just referred to as propagation) is a process in which a reactive intermediate is continuously regenerated during the course of a Chain reaction#Chemical chain reactions, chemical chain reaction. For exa ...
. Alternatively the phenyl radical can abstract any loose proton from 7 forming the arene 8 in a chain termination
In polymer chemistry, chain termination is any chemical reaction that ceases the formation of reactive intermediates in a chain propagation step in the course of a polymerization, effectively bringing it to a halt.
Mechanisms of termination ...
reaction.
The involvement of a radical intermediate in a new type of nucleophilic aromatic substitution
A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic c ...
was invoked when the product distribution was compared between a certain aromatic chloride and an aromatic iodide in reaction with potassium amide. The chloride reaction proceeds through a classical aryne
In organic chemistry, arynes and benzynes are a class of highly Reactivity (chemistry), reactive chemical Chemical species, species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes (1,2-didehydro ...
intermediate:
 The
The isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s 1a and 1b form the same aryne 2 which continues to react to the aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an in ...
s 3a and 3b in a 1 to 1.5 ratio. Clear-cut ''cine''-substitution would give a 1:1 ratio, but additional steric and electronic factors come into play as well.
Replacing chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
by iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a vi ...
in the 1,2,4-trimethylbenzene moiety drastically changes the product distribution:
 It now resembles ''ipso''-substitution with 1a forming preferentially 3a and 1b forming 3b.
It now resembles ''ipso''-substitution with 1a forming preferentially 3a and 1b forming 3b. Radical scavenger A scavenger in chemistry is a chemical substance added to a mixture in order to remove or de-activate impurities and unwanted reaction products, for example oxygen, to make sure that they will not cause any unfavorable reactions. Their use is wide- ...
s suppress ''ipso''-substitution in favor of ''cine''-substitution and the addition of potassium metal as an electron donor and radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical in ...
does exactly the opposite.''Alkali metal promoted aromatic "nucleophilic" substitution'' Joseph F. Bunnett and Jhong Kook Kim ''J. Am. Chem. Soc.'' 1970, ''92'', 7464 – 7466. ()
See also
*Birch reduction
The Birch reduction or Metal-Ammonia reduction is an organic reaction that is used to convert arenes to Cyclohexa-1,4-diene, 1,4-cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch (organic chemist), Arthur Birch and i ...
*Nucleophilic aromatic substitution
A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic c ...
References
{{Organic reactions Free radical reactions Reaction mechanisms