quintuple bond on:
[Wikipedia]
[Google]
[Amazon]
 A quintuple bond in
A quintuple bond in 

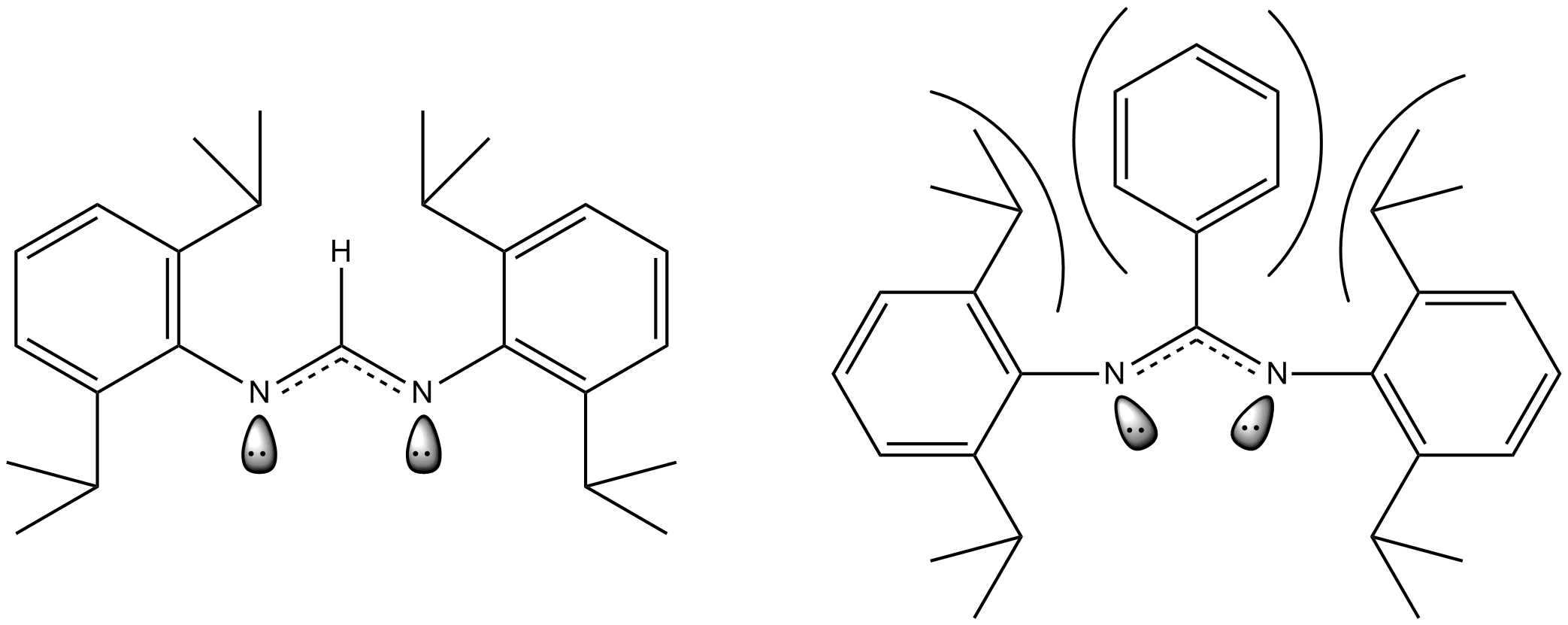 The above example shows the ligand used in the dimolybdenum complex shown earlier. When the carbon between the two nitrogens in the ligand has a hydrogen bound to it, the steric repulsion is small. However, when the hydrogen is replaced with a much more bulky phenyl ring the steric repulsion increases dramatically and the ligand "bows" which causes a change in the orientation of the lone pairs of electrons on the nitrogen atoms. These lone pairs are what is responsible for forming bonds with the metal centers so forcing them to move closer together also forces the metal centers to be positioned closer together. Thus, decreasing the length of the quintuple bond. In the case where this ligand is bound to quintuply bonded dimolybdenum the quintuple bond length goes from 201.87 pm to 201.57 pm when the hydrogen in replaced with a phenyl group. Similar results have also been demonstrated in dichromium quintuple bond complexes as well.
The above example shows the ligand used in the dimolybdenum complex shown earlier. When the carbon between the two nitrogens in the ligand has a hydrogen bound to it, the steric repulsion is small. However, when the hydrogen is replaced with a much more bulky phenyl ring the steric repulsion increases dramatically and the ligand "bows" which causes a change in the orientation of the lone pairs of electrons on the nitrogen atoms. These lone pairs are what is responsible for forming bonds with the metal centers so forcing them to move closer together also forces the metal centers to be positioned closer together. Thus, decreasing the length of the quintuple bond. In the case where this ligand is bound to quintuply bonded dimolybdenum the quintuple bond length goes from 201.87 pm to 201.57 pm when the hydrogen in replaced with a phenyl group. Similar results have also been demonstrated in dichromium quintuple bond complexes as well.
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
is an unusual type of chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
, first reported in 2005 for a dichromium compound. Single bonds, double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
s, and triple bond
A triple bond in chemistry is a chemical bond between two atoms involving six Electron pair bond, bonding electrons instead of the usual two in a covalent bond, covalent single bond. Triple bonds are stronger than the equivalent covalent bond, sin ...
s are commonplace in chemistry. Quadruple bonds are rarer and are currently known only among the transition metals, especially for Cr, Mo, W, and Re, e.g. o2Cl8sup>4− and e2Cl8sup>2−. In a quintuple bond, ten electrons participate in bonding between the two metal centers, allocated as σ2π4δ4.
In some cases of high-order bonds between metal atoms, the metal-metal bonding is facilitated by ligands that link the two metal centers and reduce the interatomic distance. By contrast, the chromium dimer with quintuple bonding is stabilized by a bulky terphenyl (2,6- 2,6-diisopropyl)phenylhenyl) ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s. The species is stable up to 200 °C. The chromium–chromium quintuple bond has been analyzed with multireference ''ab initio'' and DFT methods, which were also used to elucidate the role of the terphenyl ligand, in which the flanking aryls were shown to interact very weakly with the chromium atoms, causing only a small weakening of the quintuple bond. A 2007 theoretical study identified two global minima for quintuple bonded RMMR compounds: a ''trans''-bent molecular geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that det ...
and surprisingly another ''trans''-bent geometry with the R substituent in a bridging position.
In 2005, a quintuple bond was postulated to exist in the hypothetical uranium
Uranium is a chemical element; it has chemical symbol, symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Ura ...
molecule U2 based on computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of mol ...
. Diuranium compounds are rare, but do exist; for example, the anion.
In 2007 the shortest-ever metal–metal bond (180.28 pm) was reported to exist also in a compound containing a quintuple chromium-chromium bond with diazadiene bridging ligands. Other metal–metal quintuple bond containing complexes that have been reported include quintuply bonded dichromium with -(2,4,6-triisopropylphenyl)pyridin-2-yl2,4,6-trimethylphenyl)amine bridging ligands and a dichromium complex with amidinate bridging ligands.
Synthesis of quintuple bonds is usually achieved through reduction of a dimetal species using potassium graphite. This adds valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s to the metal centers, giving them the needed number of electrons to participate in quintuple bonding. Below is a figure of a typical quintuple bond synthesis.
:
Dimolybdenum quintuple bonds
In 2009 a dimolybdenum compound with a quintuple bond and two di amido bridging ligands was reported with a Mo–Mo bond length of 202 pm. The compound was synthesised starting frompotassium octachlorodimolybdate
Potassium octachlorodimolybdate (systematically named potassium bis(tetrachloromolybdate)(''Mo''–''Mo'')(4−)) is an inorganic compound with the chemical formula . It is known as a red-coloured, microcrystalline solid. The anion is of historic ...
(which already contains a Mo2 quadruple bond) and a lithium amidinate, followed by reduction with potassium graphite:
:
Bonding
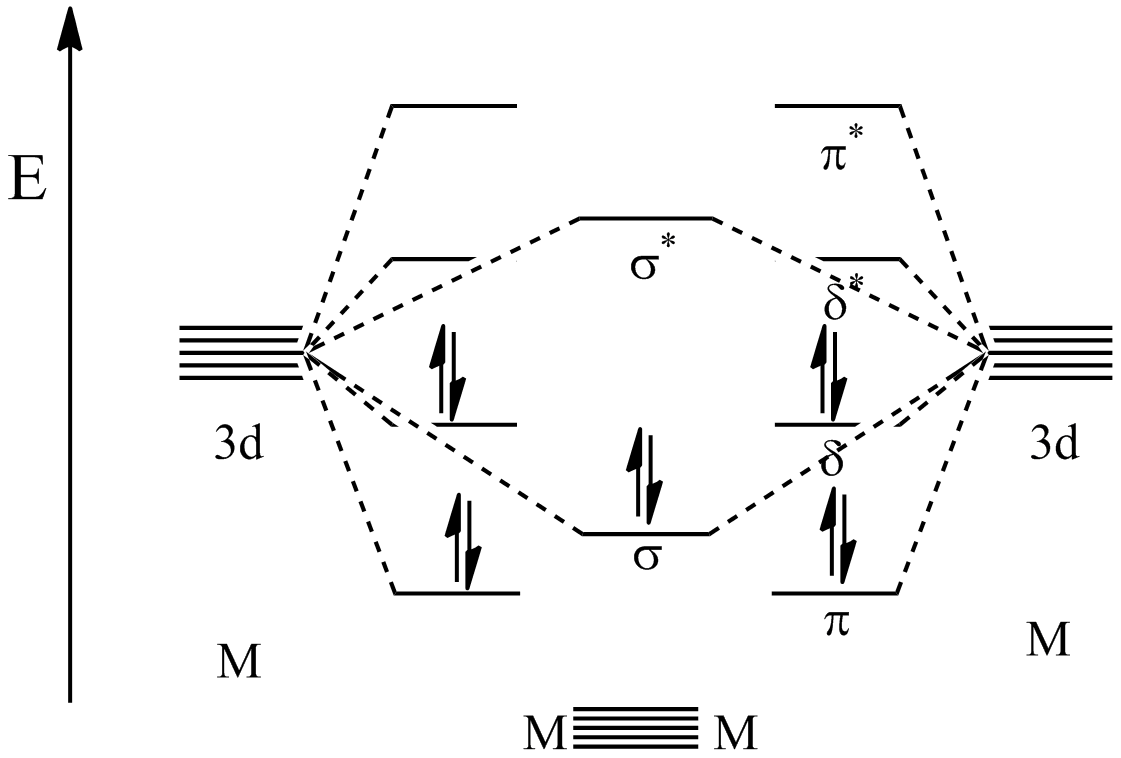
As stated above, metal–metal quintuple bonds have a σ2π4δ4 configuration. Among the five bonds present between the metal centers, one is asigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
, two are pi bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
s, and two are delta bond
In chemistry, a delta bond (δ bond) is a Covalent bond, covalent chemical bond, in which four lobes of an atomic orbital on one atom orbital overlap, overlap four lobes of an atomic orbital on another atom. This overlap leads to the formation o ...
s. The σ-bond is the result of mixing between the d''z''2 orbital on each metal center. The first π-bond comes from mixing of the d''yz'' orbitals from each metal while the other π-bond comes from the d''xz'' orbitals on each metal mixing. Finally the δ-bonds come from mixing of the d''xy'' orbitals as well as mixing between the d''x''2−''y''2 orbitals from each metal.
Molecular orbital calculations have elucidated the relative energies of the orbitals created by these bonding interactions. As shown in the figure below, the lowest energy orbitals are the π bonding orbitals followed by the σ bonding orbital. The next highest are the δ bonding orbitals which represent the HOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called ...
. Because the 10 valence electrons of the metals are used to fill these first 5 orbitals, the next highest orbital becomes the LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
which is the δ* antibonding orbital. Though the π and δ orbitals are represented as being degenerate, they in fact are not. This is because the model shown here is a simplification and that hybridization of s, p, and d orbitals is believed to take place, causing a change in the orbital energy levels.

Ligand role in metal–metal quintuple bond length
Quintuple bond lengths are heavily dependent on the ligands bound to the metal centers. Nearly all complexes containing a metal–metal quintuple bond have bidentate bridging ligands, and even those that do not, such as the terphenyl complex mentioned earlier, have some bridging characteristic to it through metal– ipso-carbon interactions. The bidentate ligand can act as a sort of tweezer in that in order forchelation
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
to occur the metal atoms must move closer together, thereby shortening the quintuple bond length. The two ways in which to obtain shorter metal–metal distances is to either reduce the distance between the chelating atoms in the ligand by changing the structure, or by using steric effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape (conformational isomerism, co ...
to force a conformational change in the ligand that bends the molecule in a way that forces the chelating atoms closer together. An example of the latter is shown below:
: The above example shows the ligand used in the dimolybdenum complex shown earlier. When the carbon between the two nitrogens in the ligand has a hydrogen bound to it, the steric repulsion is small. However, when the hydrogen is replaced with a much more bulky phenyl ring the steric repulsion increases dramatically and the ligand "bows" which causes a change in the orientation of the lone pairs of electrons on the nitrogen atoms. These lone pairs are what is responsible for forming bonds with the metal centers so forcing them to move closer together also forces the metal centers to be positioned closer together. Thus, decreasing the length of the quintuple bond. In the case where this ligand is bound to quintuply bonded dimolybdenum the quintuple bond length goes from 201.87 pm to 201.57 pm when the hydrogen in replaced with a phenyl group. Similar results have also been demonstrated in dichromium quintuple bond complexes as well.
The above example shows the ligand used in the dimolybdenum complex shown earlier. When the carbon between the two nitrogens in the ligand has a hydrogen bound to it, the steric repulsion is small. However, when the hydrogen is replaced with a much more bulky phenyl ring the steric repulsion increases dramatically and the ligand "bows" which causes a change in the orientation of the lone pairs of electrons on the nitrogen atoms. These lone pairs are what is responsible for forming bonds with the metal centers so forcing them to move closer together also forces the metal centers to be positioned closer together. Thus, decreasing the length of the quintuple bond. In the case where this ligand is bound to quintuply bonded dimolybdenum the quintuple bond length goes from 201.87 pm to 201.57 pm when the hydrogen in replaced with a phenyl group. Similar results have also been demonstrated in dichromium quintuple bond complexes as well.
Research trends
Efforts continue to prepare shorter quintuple bonds. Quintuple-bonded dichromium complexes appear to act like magnesium to produceGrignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
s.
References
See also
{{Chemical bonding theory Chemical bonding