Polystyrene sulfonic acid on:
[Wikipedia]
[Google]
[Amazon]
Polystyrene sulfonates are a group of medications used to treat
 Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and chronic kidney disease for people with
Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and chronic kidney disease for people with
high blood potassium
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0mmol/L (3.5 and 5.0mEq/L) with levels above 5.5mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasi ...
. Effects generally take hours to days. They are also used to remove potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmosph ...
, calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar t ...
, and sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable ...
from solutions in technical applications.
Common side effects include loss of appetite, gastrointestinal upset, constipation, and low blood calcium. These polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
s are derived from polystyrene by the addition of sulfonate
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-o ...
functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the re ...
s.
Sodium polystyrene sulfonate was approved for medical use in the United States in 1958.
A polystyrene sulfonate was developed in the 2000s to treat Clostridium ''difficile'' associated diarrhea under the name Tolevamer, but it was never marketed.
Medical uses
 Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and chronic kidney disease for people with
Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and chronic kidney disease for people with hyperkalemia
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0mmol/L (3.5 and 5.0mEq/L) with levels above 5.5mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasi ...
(abnormal high blood serum potassium levels). However, it is unclear if it is beneficial and there is concern about possible side effects when it is combined with sorbitol.
Polystyrene sulfonates are given by mouth with a meal or rectal
The rectum is the final straight portion of the large intestine in humans and some other mammals, and the gut in others. The adult human rectum is about long, and begins at the rectosigmoid junction (the end of the sigmoid colon) at the le ...
ly by retention enema
An enema, also known as a clyster, is an injection of fluid into the lower bowel by way of the rectum.Cullingworth, ''A Manual of Nursing, Medical and Surgical'':155 The word enema can also refer to the liquid injected, as well as to a device ...
.
Side effects
Intestinal disturbances are common, including loss of appetite,nausea
Nausea is a diffuse sensation of unease and discomfort, sometimes perceived as an urge to vomit. While not painful, it can be a debilitating symptom if prolonged and has been described as placing discomfort on the chest, abdomen, or back of the ...
, vomiting
Vomiting (also known as emesis and throwing up) is the involuntary, forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose.
Vomiting can be the result of ailments like food poisoning, gastroenteri ...
, and constipation
Constipation is a bowel dysfunction that makes bowel movements infrequent or hard to pass. The stool is often hard and dry. Other symptoms may include abdominal pain, bloating, and feeling as if one has not completely passed the bowel movement ...
. In rare cases, it has been associated with colonic necrosis. Changes in electrolyte blood levels such as hypomagnesemia
Magnesium deficiency is an electrolyte disturbance in which there is a low level of magnesium in the body. It can result in multiple symptoms. Symptoms include tremor, poor coordination, muscle spasms, loss of appetite, personality changes, an ...
, hypocalcemia
Hypocalcemia is a medical condition characterized by low calcium levels in the blood serum. The normal range of blood calcium is typically between 2.1–2.6 mmol/L (8.8–10.7 mg/dL, 4.3–5.2 mEq/L) while levels less than 2.1 mm ...
, and hypokalemia
Hypokalemia is a low level of potassium (K+) in the blood serum. Mild low potassium does not typically cause symptoms. Symptoms may include feeling tired, leg cramps, weakness, and constipation. Low potassium also increases the risk of an abno ...
may occur. Polystyrene sulfonates should not be used in people with obstructive bowel disease and in newborns with reduced gut motility
Peristalsis ( , ) is a radially symmetrical contraction and relaxation of muscles that propagate in a wave down a tube, in an anterograde direction. Peristalsis is progression of coordinated contraction of involuntary circular muscles, which ...
. for Kayexalate.
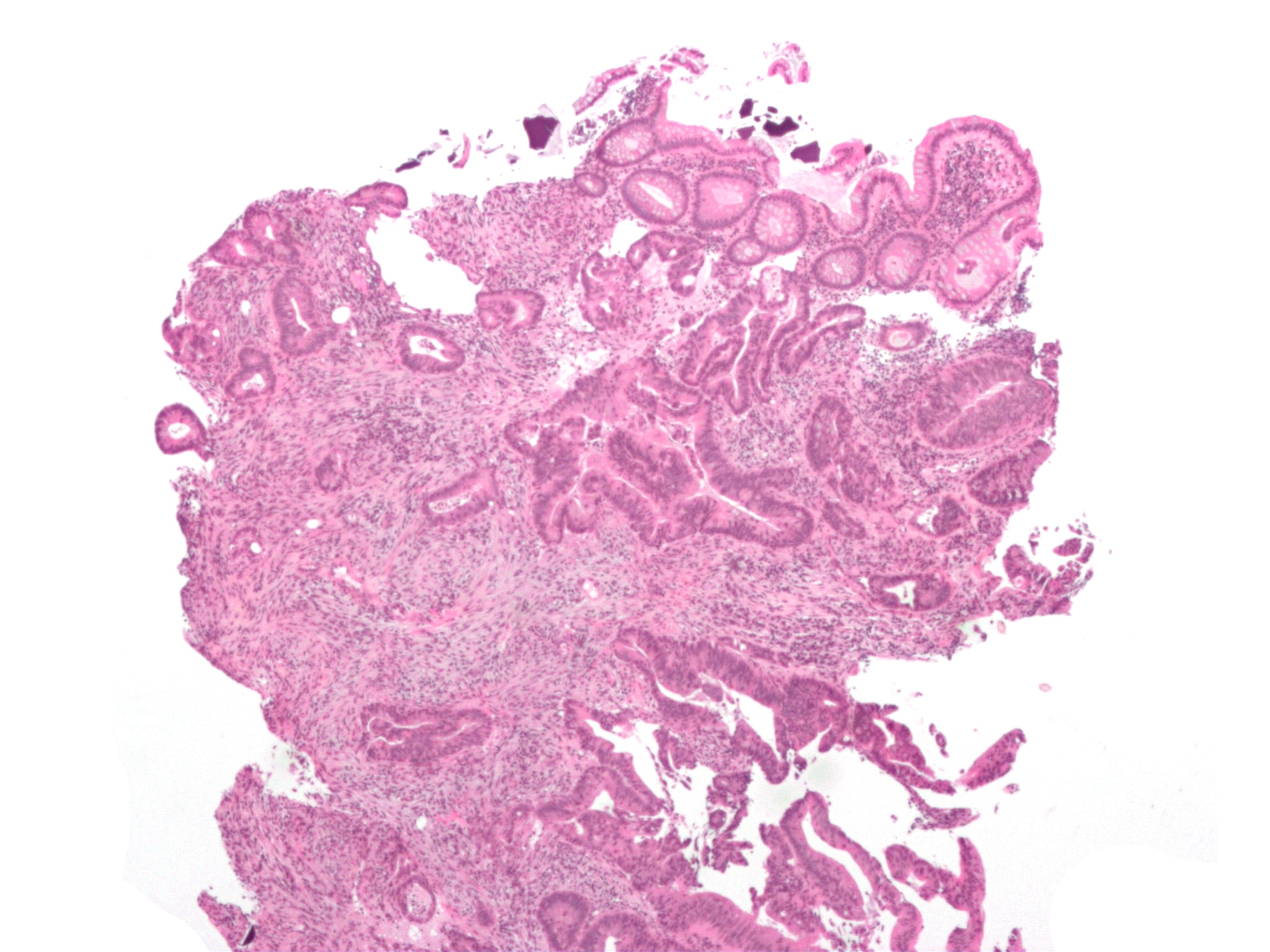
Intestinal injury
A total of 58 cases of intestinal injury including necrosis of the colon have been reported with polystyrene sulfonate as of 2013. Well more cases have been reported when used in combination with sorbitol and other cases have occurred when used alone.Interactions
Polystyrene sulfonates can bind to various drugs within the digestive tract and thus lower their absorption and effectiveness. Common examples includelithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid ...
, thyroxine
File:Thyroid_system.svg, upright=1.5, The thyroid system of the thyroid hormones T3 and T4
rect 376 268 820 433 Thyroid-stimulating hormone
rect 411 200 849 266 Thyrotropin-releasing hormone
rect 297 168 502 200 Hypothalamus
rect 66 216 386 25 ...
, and digitalis
''Digitalis'' ( or ) is a genus of about 20 species of herbaceous perennial plants, shrubs, and biennials, commonly called foxgloves.
''Digitalis'' is native to Europe, western Asia, and northwestern Africa. The flowers are tubular in shap ...
. In September 2017, the FDA recommended separating the dosing of polystyrene sulfonate from any other oral medications by at least three hours to avoid any potential interactions.
Mechanism of action
Hyperkalemia
Polystyrene sulfonates release sodium or calcium ions in the stomach in exchange for hydrogen ions. When the resin reaches the large intestine the hydrogen ions are exchanged for free potassium ions; the resin is then eliminated in the feces. The net effect is lowering the amount of potassium available for absorption into the blood and increasing the amount that is excreted via the feces. The effect is a reduction of potassium levels in the body, at a capacity of 1 mEq of potassium exchanged per 1 g of resin.Production and chemical structure
Polystyrene sulfonic acid, the acid whose salts are the polystyrene sulfonates, has the idealized formula (CH2CHC6H4SO3H)''n''. The material is prepared bysulfonation Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution. Aryl sulfonic acids are used as detergents, dye, and drugs.
Stoichiometry a ...
of polystyrene:
:(CH2CHC6H5)''n'' + ''n'' SO3 → (CH2CHC6H4SO3H)''n''
Several methods exist for this conversion, which can lead to varying degree of sulfonation. Usually the polystyrene is crosslinked
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
, which keeps the polymer from dissolving. Since the sulfonic acid group (SO3H) is strongly acidic, this polymer neutralizes bases. In this way, various salts of the polymer can be prepared, leading to sodium, calcium, and other salts:
:(CH2CHC6H4SO3H)''n'' + ''n'' NaOH → (CH2CHC6H4SO3Na)''n'' + ''n'' H2O
These ion-containing polymers are called ionomer
An ionomer () ('' iono-'' + ''-mer'') is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent ...
s.
Alternative sulfonation methods
Double substitutions of the phenyl rings are known to occur, even with conversions well below 100%. Crosslinking reactions are also found, where condensation of two sulfonic acid groups yields a sulfonyl crosslink. On the other hand, the use of milder conditions such as acetyl sulfate leads to incomplete sulfonation. Recently, the atom transfer radical polymerization (ATRP) of protected styrene sulfonates has been reported, leading to well defined linear polymers, as well as more complicated molecular architectures.Chemical uses
Polystyrene sulfonates are useful because of theirion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
properties. Linear ionic polymers are generally water-soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
, whereas cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ed materials (called resin
In polymer chemistry and materials science, resin is a solid or highly viscous substance of plant or synthetic origin that is typically convertible into polymers. Resins are usually mixtures of organic compounds. This article focuses on n ...
s) do not dissolve in water. These polymers are classified as polysalts and ionomer
An ionomer () ('' iono-'' + ''-mer'') is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent ...
s.
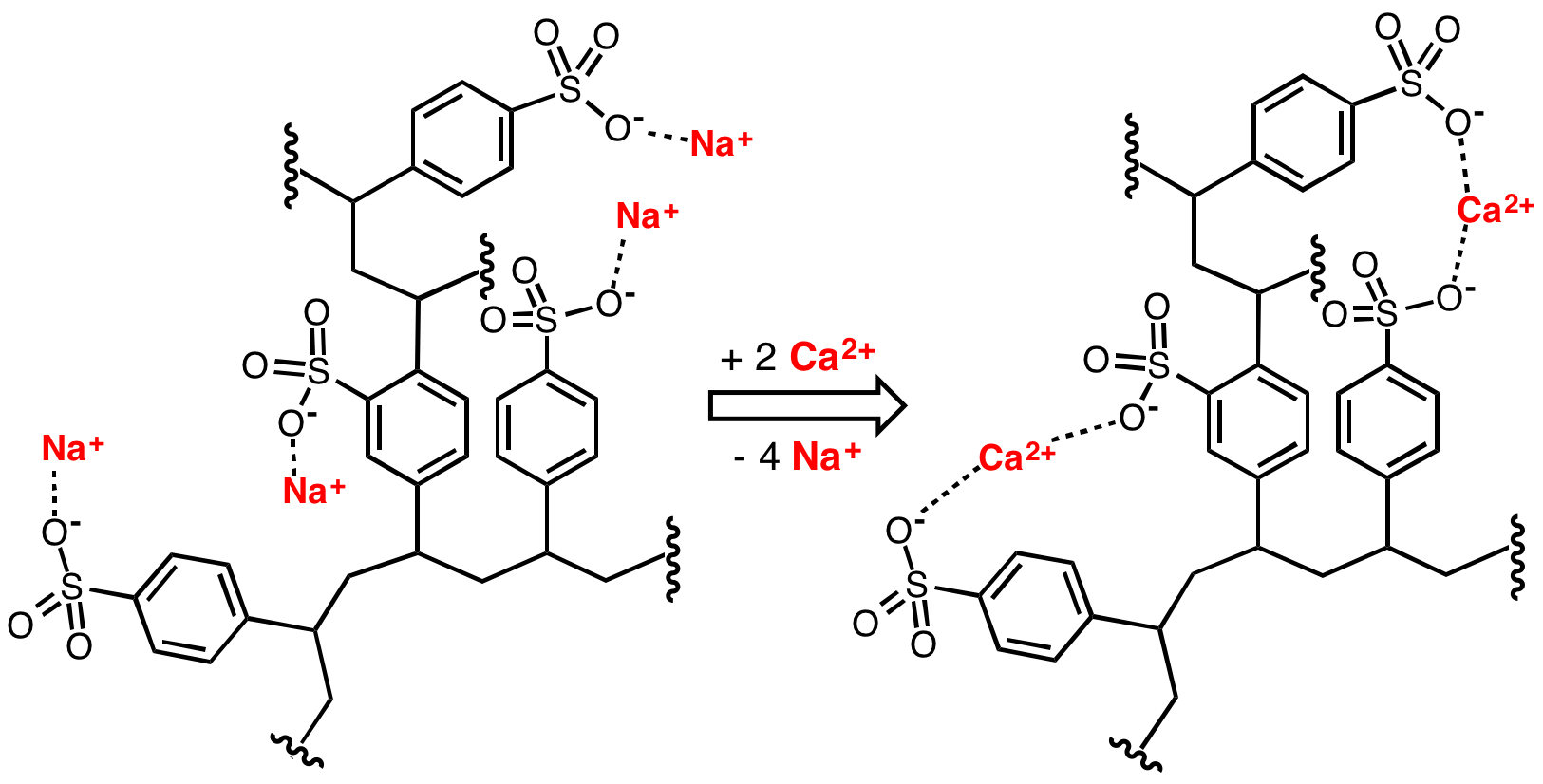
Water softening
Water softening is achieved by percolatinghard water
Hard water is water that has high mineral content (in contrast with "soft water"). Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbo ...
through a bed of the sodium form of cross-linked polystyrene sulfonate. The hard ions such as calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar t ...
(Ca2+) and magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
(Mg2+) adhere to the sulfonate groups, displacing sodium ions. The resulting solution of sodium ions is softened.

Other uses
Sodium polystyrene sulfonate is used as a superplastifier in cement, as a dye improving agent for cotton, and as proton exchange membranes in fuel cell applications. In its acid form, the resin is used as a solid acid catalyst in organic synthesis.References
{{Drugs for treatment of hyperkalemia and hyperphosphatemia Benzenesulfonates Nephrology procedures Organic polymers Plastics Polyelectrolytes Chelating agents used as drugs Acid catalysts Vinyl polymers Sanofi