Phthalocyanine on:
[Wikipedia]
[Google]
[Amazon]
Phthalocyanine () is a large,
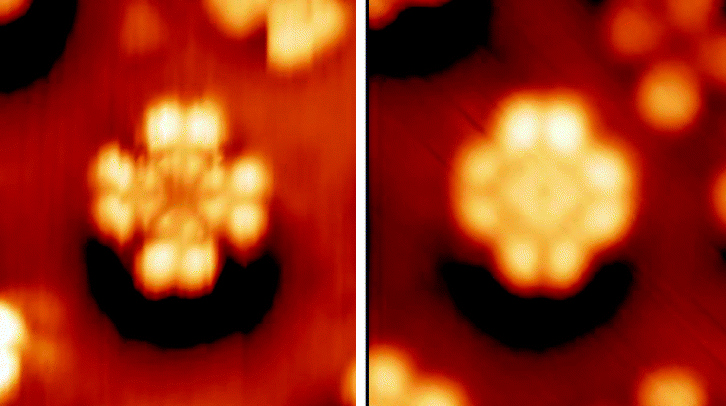 Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents.
Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents.
aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
, macrocyclic
Macrocycles are often described as molecules and ions containing a ring of twelve or more atoms. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.
...
, organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
with the formula and is of theoretical or specialized interest in chemical dyes and photoelectricity.
It is composed of four isoindole units linked by a ring of nitrogen atoms. = has a two-dimensional geometry and a ring system consisting of 18 π-electrons. The extensive delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
of the π-electrons affords the molecule useful properties, lending itself to applications in dyes and pigments. Metal complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es derived from , the conjugate base of , are valuable in catalysis, organic solar cells
An organic solar cell (OSC) or plastic solar cell is a type of photovoltaic that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules, for light absorption and charge transport t ...
, and photodynamic therapy.
Properties
 Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents.
Phthalocyanine and derived metal complexes (MPc) tend to aggregate and, thus, have low solubility in common solvents.
Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms ...
at 40 °C dissolves less than a milligram of or CuPc per litre. and CuPc dissolve easily in sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
due to the protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
of the nitrogen atoms bridging the pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
rings. Many phthalocyanine compounds are, thermally, very stable and do not melt but can be sublimed. CuPc sublimes at above 500 °C under inert gases (nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
, ). Substituted phthalocyanine complexes often have much higher solubility. They are less thermally stable and often can not be sublimed. Unsubstituted phthalocyanines strongly absorb light between 600 and 700 nm, thus these materials are blue or green. Substitution can shift the absorption towards longer wavelengths, changing color from pure blue to green to colorless (when the absorption is in the near infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from arou ...
).
There are many derivatives of the parent phthalocyanine, where either carbon atoms of the macrocycle are exchanged for nitrogen atoms or the peripheral hydrogen atoms are substituted by functional groups like halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this grou ...
s, hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
, amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent ...
, alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloa ...
, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
, thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
, alkoxy
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also ...
and nitrosyl groups. These modifications allow for the tuning of the electrochemical properties of the molecule such as absorption and emission wavelengths and conductance.
History
In 1907, an unidentified blue compound, now known to be phthalocyanine, was reported. In 1927, Swiss researchers serendipitously discovered copper phthalocyanine, coppernaphthalocyanine
Naphthalocyanine is a cross-shaped organic molecule consisting of 48 carbon, 8 nitrogen and 26 hydrogen atoms, it is a derivative of phthalocyanine. IBM Research
IBM Research is the research and development division for IBM, an American multin ...
, and copper octamethylphthalocyanine in an attempted conversion of ''o''-dibromobenzene into phthalonitrile. They remarked on the enormous stability of these complexes but did not further characterize them. In the same year, iron phthalocyanine was discovered at Scottish Dyes of Grangemouth
Grangemouth ( sco, Grangemooth; gd, Inbhir Ghrainnse, ) is a town in the Falkirk council area, Scotland. Historically part of the county of Stirlingshire, the town lies in the Forth Valley, on the banks of the Firth of Forth, east of Falkir ...
, Scotland (later ICI). It was not until 1934 that Sir Patrick Linstead characterized the chemical and structural properties of iron phthalocyanine.
Synthesis
Phthalocyanine is formed through the cyclotetramerization of variousphthalic acid
Phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale. Phthalic acid ...
derivatives including phthalonitrile, diiminoisoindole, phthalic anhydride
Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commer ...
, and phthalimides
Phthalimide is the organic compound with the formula C6H4(CO)2NH. It is the imide derivative of phthalic anhydride. It is a sublimable white solid that is slightly soluble in water but more so upon addition of base. It is used as a precursor ...
. Alternatively, heating phthalic anhydride
Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commer ...
in the presence of urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
yields .
Using such methods, approximately 57,000 tonnes (63,000 Imperial tons) of various phthalocyanines were produced in 1985. More often, MPc is synthesized rather than due to the greater research interest in the former. To prepare these complexes, the phthalocyanine synthesis is conducted in the presence of metal salts. Two copper phthalocyanines are shown in the figure below.
:
Halogenated and sulfonated Aromatic sulfonation is an organic reaction in which a hydrogen atom on an arene is replaced by a sulfonic acid functional group in an electrophilic aromatic substitution. Aryl sulfonic acids are used as detergents, dye, and drugs.
Stoichiometry an ...
derivatives of copper phthalocyanines are commercially important as dyes. Such compounds are prepared by treating CuPc with chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
, bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
or oleum
Oleum (Latin ''oleum'', meaning oil), or fuming sulfuric acid, is a term referring to solutions of various compositions of sulfur trioxide in sulfuric acid, or sometimes more specifically to disulfuric acid (also known as pyrosulfuric acid). Ol ...
.
Applications
At the initial discovery of Pc, its uses were primarily limited to dyes and pigments. Modification of the substituents attached to the peripheral rings allows for the tuning of the absorption and emission properties of Pc to yield differently colored dyes and pigments. There has since been significant research on H2Pc and MPc resulting in a wide range of applications in areas includingphotovoltaics
Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially ...
, photodynamic therapy, nanoparticle
A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...
construction, and catalysis. The electrochemical properties of MPc make them effective electron-donors and -acceptors. As a result, MPc-based organic solar cells
An organic solar cell (OSC) or plastic solar cell is a type of photovoltaic that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules, for light absorption and charge transport t ...
with power conversion efficiencies at or below 5% have been developed. Furthermore, MPcs have been used as catalysts for the oxidation of methane, phenols, alcohols, polysaccharides, and olefins; MPcs can also be used to catalyze C–C bond formation and various reduction reactions. Silicon and zinc phthalocyanines have been developed as photosensitizers for non-invasive cancer treatment. Various MPcs have also shown the ability to form nanostructures which have potential applications in electronics and biosensing. Phthalocyanine is also used on some recordable DVDs.
Toxicity and hazards
No evidence has been reported for acute toxicity or carcinogenicity of phthalocyanine compounds. The (rats, oral) is 10 g/kg.Related compounds
Phthalocyanines are structurally related to other tetrapyrrole macrocyles including porphyrins andporphyrazine
Porphyrazines, or tetraazaporphyrins, are tetrapyrrole macrocycles similar to porphyrins and phthalocyanines. Pioneered by Sir R. Patrick Linstead as an extension of his work on phthalocyanines, porphyrazines differ from porphyrins in that they c ...
s. They feature four pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-meth ...
-like subunits linked to form a 16 membered inner ring composed of alternating carbon and nitrogen atoms. Structurally larger analogues include naphthalocyanines. The pyrrole-like rings within are closely related to isoindole. Both porphyrins and phthalocyanines function as planar tetradentate
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands.
Tetradentate ligands ar ...
dianionic ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
s that bind metals through four inwardly projecting nitrogen centers. Such complexes are formally derivatives of , the conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
of .
Footnotes
References
External links
* * * * {{Authority control Macrocycles Chelating agents