partial molar volume on:
[Wikipedia]
[Google]
[Amazon]
In
 The partial
The partial Wolfram Mathworld: Euler's homogeneous function theorem
/ref> : where is the partial molar of component defined as: : By Euler's second theorem for homogeneous functions, is a homogeneous function of degree 0 (i.e., is an intensive property) which means that for any : : In particular, taking where , one has : where is the concentration expressed as the
mixtures, partial molar quantities, and ideal solutions
su
/nowiki>]
On-line calculator for densities and partial molar volumes of aqueous solutions of some common electrolytes and their mixtures, at temperatures up to 323.15 K.
Physical chemistry Thermodynamic properties Chemical thermodynamics
thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of ther ...
, a partial molar property is a quantity which describes the variation of an extensive property
Physical properties of materials and systems can often be categorized as being either intensive or extensive, according to how the property changes when the size (or extent) of the system changes. According to IUPAC, an intensive quantity is one ...
of a solution or mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which are not chemically bonded. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the ...
with changes in the molar composition of the mixture at constant temperature and pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and ...
. It is the partial derivative
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant (as opposed to the total derivative, in which all variables are allowed to vary). Par ...
of the extensive property with respect to the amount
Quantity or amount is a property that can exist as a multitude or magnitude, which illustrate discontinuity and continuity. Quantities can be compared in terms of "more", "less", or "equal", or by assigning a numerical value multiple of a unit ...
(number of moles) of the component of interest. Every extensive property of a mixture has a corresponding partial molar property.
Definition
 The partial
The partial molar volume
In chemistry and related fields, the molar volume, symbol ''V''m, or \tilde V of a substance is the ratio of the volume occupied by a substance to the amount of substance, usually given at a given temperature and pressure. It is equal to the mola ...
is broadly understood as the contribution that a component of a mixture makes to the overall volume of the solution. However, there is more to it than this:
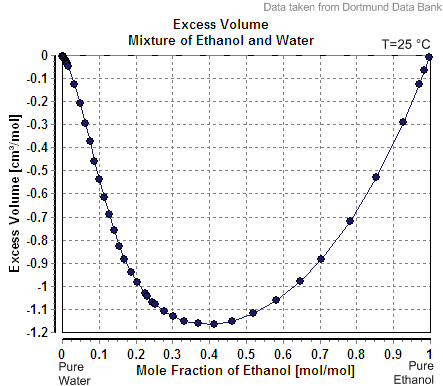
When one mole of water is added to a large volume of water at 25 °C, the volume increases by 18 cm3. The molar volume of pure water would thus be reported as 18 cm3 mol−1. However, addition of one mole of water to a large volume of pure ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hyd ...
results in an increase in volume of only 14 cm3. The reason that the increase is different is that the volume occupied by a given number of water molecules depends upon the identity of the surrounding molecules. The value 14 cm3 is said to be the partial molar volume of water in ethanol.
In general, the partial molar volume of a substance X in a mixture is the change in volume per mole of X added to the mixture.
The partial molar volumes of the components of a mixture vary with the composition of the mixture, because the environment of the molecules in the mixture changes with the composition. It is the changing molecular environment (and the consequent alteration of the interactions between molecules) that results in the thermodynamic properties of a mixture changing as its composition is altered.
If, by , one denotes a generic extensive property of a mixture, it will always be true that it depends on the pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and ...
(), temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
(), and the amount of each component of the mixture (measured in moles, ''n''). For a mixture with ''q'' components, this is expressed as
:
Now if temperature ''T'' and pressure ''P'' are held constant, is a homogeneous function
In mathematics, a homogeneous function is a function of several variables such that, if all its arguments are multiplied by a scalar, then its value is multiplied by some power of this scalar, called the degree of homogeneity, or simply the ''d ...
of degree 1, since doubling the quantities of each component in the mixture will double . More generally, for any :
:
By Euler's first theorem for homogeneous functions, this implies/ref> : where is the partial molar of component defined as: : By Euler's second theorem for homogeneous functions, is a homogeneous function of degree 0 (i.e., is an intensive property) which means that for any : : In particular, taking where , one has : where is the concentration expressed as the
mole fraction
In chemistry, the mole fraction or molar fraction (''xi'' or ) is defined as unit of the amount of a constituent (expressed in moles), ''ni'', divided by the total amount of all constituents in a mixture (also expressed in moles), ''n''tot. This ...
of component .
Since the molar fractions satisfy the relation
:
the ''xi'' are not independent, and the partial molar property is a function of only mole fractions:
:
The partial molar property is thus an intensive property
Physical properties of materials and systems can often be categorized as being either intensive or extensive, according to how the property changes when the size (or extent) of the system changes. According to IUPAC, an intensive quantity is one ...
- it does not depend on the size of the system.
The partial volume is not the partial molar volume.
Applications
Partial molar properties are useful because chemicalmixture
In chemistry, a mixture is a material made up of two or more different chemical substances which are not chemically bonded. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the ...
s are often maintained at constant temperature and pressure and under these conditions, the value of any extensive property
Physical properties of materials and systems can often be categorized as being either intensive or extensive, according to how the property changes when the size (or extent) of the system changes. According to IUPAC, an intensive quantity is one ...
can be obtained from its partial molar property. They are especially useful when considering specific properties of pure substances (that is, properties of one mole of pure substance) and properties of mixing (such as the heat of mixing
In thermodynamics, the enthalpy of mixing (also heat of mixing and excess enthalpy) is the enthalpy liberated or absorbed from a substance upon mixing. When a substance or compound is combined with any other substance or compound, the enthalpy of ...
or entropy of mixing
In thermodynamics, the entropy of mixing is the increase in the total entropy when several initially separate systems of different composition, each in a thermodynamic state of internal equilibrium, are mixed without chemical reaction by the therm ...
). By definition, properties of mixing are related to those of the pure substances by:
:
Here denotes a pure substance, the mixing property, and corresponds to the specific property under consideration. From the definition of partial molar properties,
:
substitution yields:
:
So from knowledge of the partial molar properties, deviation of properties of mixing from single components can be calculated.
Relationship to thermodynamic potentials
Partial molar properties satisfy relations analogous to those of the extensive properties. For theinternal energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinet ...
''U'', enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant p ...
''H'', Helmholtz free energy
In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature (isothermal). The change in the Helmholtz energy ...
''A'', and Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and p ...
''G'', the following hold:
:
:
:
where is the pressure, the volume
Volume is a measure of occupied three-dimensional space. It is often quantified numerically using SI derived units (such as the cubic metre and litre) or by various imperial or US customary units (such as the gallon, quart, cubic inch). The def ...
, the temperature, and the entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
.
Differential form of the thermodynamic potentials
The thermodynamic potentials also satisfy : : : : where is thechemical potential
In thermodynamics, the chemical potential of a species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potential of a species ...
defined as (for constant nj with j≠i):
:
This last partial derivative
In mathematics, a partial derivative of a function of several variables is its derivative with respect to one of those variables, with the others held constant (as opposed to the total derivative, in which all variables are allowed to vary). Par ...
is the same as , the partial molar Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and p ...
. This means that the partial molar Gibbs free energy and the chemical potential, one of the most important properties in thermodynamics and chemistry, are the same quantity. Under isobaric
Isobar may refer to:
* Isobar (meteorology), a line connecting points of equal atmospheric pressure reduced to sea level on the maps.
* Isobaric process, a process taking place at constant pressure
* Isobar (nuclide)
Isobars are atoms (nucl ...
(constant ''P'') and isothermal
In thermodynamics, an isothermal process is a type of thermodynamic process in which the temperature ''T'' of a system remains constant: Δ''T'' = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and ...
(constant ''T '') conditions, knowledge of the chemical potentials, , yields every property of the mixture as they completely determine the Gibbs free energy.
Measuring partial molar properties
To measure the partial molar property of a binary solution, one begins with the pure component denoted as and, keeping the temperature and pressure constant during the entire process, add small quantities of component ; measuring after each addition. After sampling the compositions of interest one can fit a curve to the experimental data. This function will be . Differentiating with respect to will give . is then obtained from the relation: :Relation to apparent molar quantities
The relation between partial molar properties and the apparent ones can be derived from the definition of the apparent quantities and of the molality. : The relation holds also for multicomponent mixtures, just that in this case subscript i is required.See also
*Apparent molar property
In thermodynamics, an apparent molar property of a solution component in a mixture or solution is a quantity defined with the purpose of isolating the contribution of each component to the non-ideality of the mixture. It shows the change in the ...
*Ideal solution
In chemistry, an ideal solution or ideal mixture is a solution that exhibits thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of mixing is zero as is the volume change on mixing by definition; the closer to zer ...
*Excess molar quantity
In chemical thermodynamics, excess properties are properties of mixtures which quantify the non- ideal behavior of real mixtures. They are defined as the difference between the value of the property in a real mixture and the value that would exist ...
*Partial specific volume
The partial specific volume \bar, express the variation of the extensive volume of a mixture in respect to composition of the masses. It is the partial derivative of volume with respect to the mass of the component of interest.
:V=\sum _^n m_i \bar ...
*Thermodynamic activity
In chemical thermodynamics, activity (symbol ) is a measure of the "effective concentration" of a species in a mixture, in the sense that the species' chemical potential depends on the activity of a real solution in the same way that it would depen ...
References
{{reflistFurther reading
* P. Atkins and J. de Paula, "Atkins' Physical Chemistry" (8th edition, Freeman 2006), chap.5 * T. Engel and P. Reid, "Physical Chemistry" (Pearson Benjamin-Cummings 2006), p. 210 * K.J. Laidler and J.H. Meiser, "Physical Chemistry" (Benjamin-Cummings 1982), p. 184-189 * P. Rock, "Chemical Thermodynamics" (MacMillan 1969), chap.9 * Ira Levine, "Physical Chemistry" (6th edition,McGraw Hill 2009),p.125-128External links
*Lecture notes from the University of Arizona detailinmixtures, partial molar quantities, and ideal solutions
su
/nowiki>]
On-line calculator for densities and partial molar volumes of aqueous solutions of some common electrolytes and their mixtures, at temperatures up to 323.15 K.
Physical chemistry Thermodynamic properties Chemical thermodynamics