Plumbylene Structure on:
[Wikipedia]
[Google]
[Amazon]
 Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of
Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of
 Transmetallation with (CH3)3Si)2Nsub>2Pb as the Pb(II) precursor has also been used to synthesize diarylplumbylenes, disilylplumbylenes, and saturated ''N''-heterocyclic plumbylenes.
Transmetallation with (CH3)3Si)2Nsub>2Pb as the Pb(II) precursor has also been used to synthesize diarylplumbylenes, disilylplumbylenes, and saturated ''N''-heterocyclic plumbylenes.
 Alternatively, plumbylenes may be synthesized from the reductive dehalogenation of tetravalent organolead compounds (R2PbX2).
Alternatively, plumbylenes may be synthesized from the reductive dehalogenation of tetravalent organolead compounds (R2PbX2).

 The bonding and reactivity in plumbylenes are dictated by the
The bonding and reactivity in plumbylenes are dictated by the  The Pb–C bond distance was found to be 2.303 Å and the C–Pb–C angle 105.7°. Notwithstanding the different levels of theory, the larger bond angle for (C6H5)2Pb compared to can be rationalized by steric effects.
Plumbylenes occur as reactive intermediates in the formation of tetravalent
The Pb–C bond distance was found to be 2.303 Å and the C–Pb–C angle 105.7°. Notwithstanding the different levels of theory, the larger bond angle for (C6H5)2Pb compared to can be rationalized by steric effects.
Plumbylenes occur as reactive intermediates in the formation of tetravalent
 Organoplumbylenes tend to exist in an equilibrium between the monomeric and dimeric form in solution, and, due to the low dimerization energy, as either monomers or dimers in the solid state, depending on the steric bulk of substituents. Bulky substituents allow the plumbylene to exist exclusively as monomers. In the dimerization, a
Organoplumbylenes tend to exist in an equilibrium between the monomeric and dimeric form in solution, and, due to the low dimerization energy, as either monomers or dimers in the solid state, depending on the steric bulk of substituents. Bulky substituents allow the plumbylene to exist exclusively as monomers. In the dimerization, a  These diplumbenes possess a ''trans''-bent structure similar to that in lighter, non-carbon congeners (
These diplumbenes possess a ''trans''-bent structure similar to that in lighter, non-carbon congeners (
 ''N''-heterocyclic plumbylene also dimerize leading to C–H activation, existing in solution in an equilibrium between the monomer and a dimer resulting from cleavage of an aryl C–H bond and formation of Pb–C and N–H bonds.
''N''-heterocyclic plumbylene also dimerize leading to C–H activation, existing in solution in an equilibrium between the monomer and a dimer resulting from cleavage of an aryl C–H bond and formation of Pb–C and N–H bonds.

 Insertions into lead-substituent bonds can also occur.27 In the examples below, insertion is accompanied by intramolecular rearrangement to place more electron-donating heteroatoms next to the electron-deficient lead.27
Insertions into lead-substituent bonds can also occur.27 In the examples below, insertion is accompanied by intramolecular rearrangement to place more electron-donating heteroatoms next to the electron-deficient lead.27

carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
, and are considered open shell
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
species.
The first plumbylene reported was the dialkylplumbylene, Me3Si)2CHsub>2Pb, which was synthesized by Michael F. Lappert ''et al'' in 1973.
Plumbylenes may be further classified into carbon-substituted plumbylenes, plumbylenes stabilized by a group 15
, -
! colspan=2 style="text-align:left;" , ↓ Period
, -
! 2
,
, -
! 3
,
, -
! 4
,
, -
! 5
,
, -
! 6
,
, -
! 7
,
, -
, colspan="2",
----
''Legend''
A pnictogen ( or ; from "to choke" and -gen, "generator") is any ...
or 16 element, and monohalogenated plumbylenes (RPbX).
Synthesis
Plumbylenes can generally be synthesized via thetransmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
of PbX2 (where X denotes halogen) with an organolithium (RLi) or Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
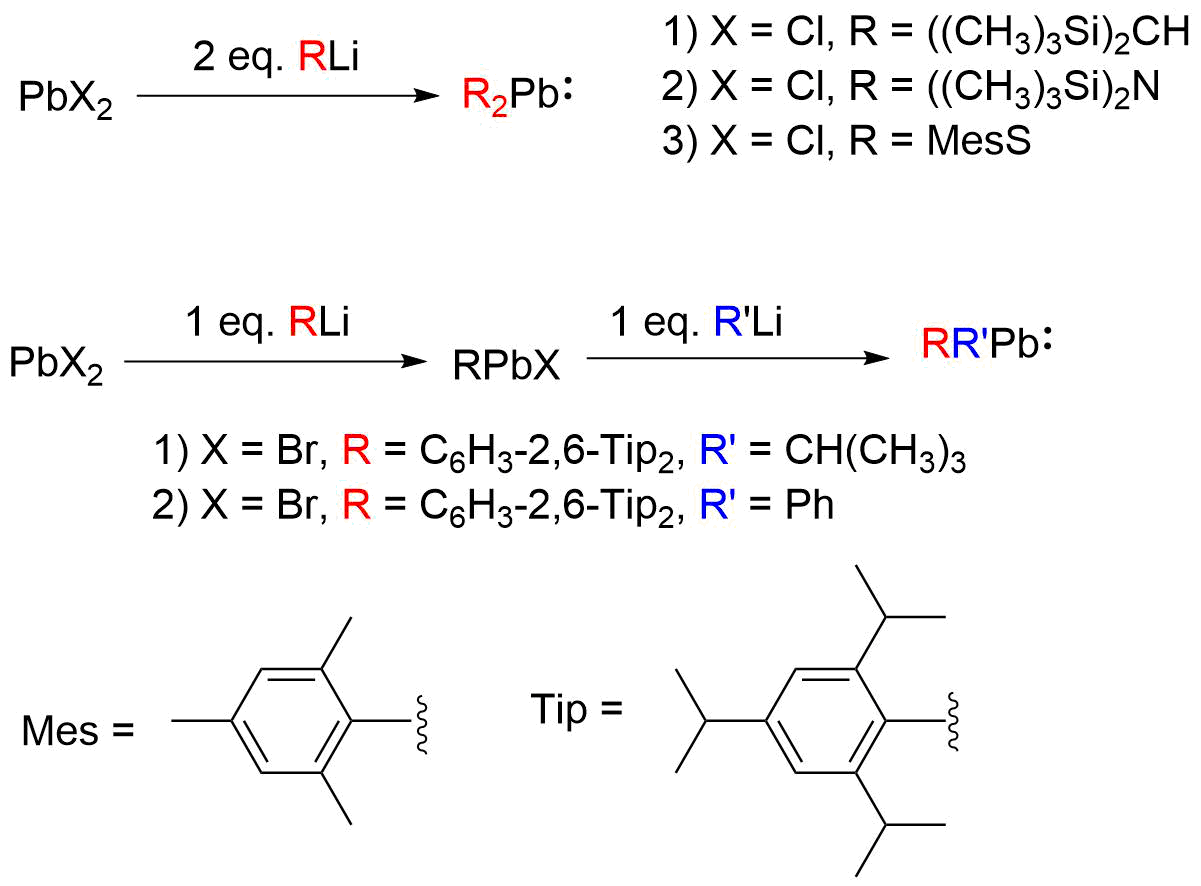
(RMgX). The first reported plumbylene, (CH3)3Si)2CHsub>2Pb, was synthesized by Michael F. Lappert ''et al'' by transmetallation of PbCl2 with (CH3)3Si)2CHi. The addition of equimolar RLi to PbX2 produces the monohalogenated plumbylene (RPbX); addition of 2 equivalents leads to disubstituted plumbylene (R2Pb). Adding an organolithium or Grignard reagent with a different organic substituent (i.e. R’Li/R’MgX) from RPbX leads to the synthesis of heteroleptic plumbylenes (RR’Pb). Dialkyl-, diaryl-, diamido-, dithioplumbylenes, and monohalogenated plumbyelenes have been successfully synthesized this way. Transmetallation with (CH3)3Si)2Nsub>2Pb as the Pb(II) precursor has also been used to synthesize diarylplumbylenes, disilylplumbylenes, and saturated ''N''-heterocyclic plumbylenes.
Transmetallation with (CH3)3Si)2Nsub>2Pb as the Pb(II) precursor has also been used to synthesize diarylplumbylenes, disilylplumbylenes, and saturated ''N''-heterocyclic plumbylenes.
 Alternatively, plumbylenes may be synthesized from the reductive dehalogenation of tetravalent organolead compounds (R2PbX2).
Alternatively, plumbylenes may be synthesized from the reductive dehalogenation of tetravalent organolead compounds (R2PbX2).

Structure and bonding
inert pair effect
The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of o ...
, whereby the combination of a widening s–p orbital energy gap
In solid-state physics, an energy gap or band gap is an energy range in a solid where no electron states exist, i.e. an energy range where the density of states vanishes.
Especially in condensed matter physics, an energy gap is often known more ab ...
as a trend down the group 14
The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern IUPAC notation, it is called group 14. In the field of semi ...
elements and a strong relativistic contraction of the 6s orbital limits sp hybridization. The 6s orbital is deep in energy and inert. Consequently, plumbylenes exclusively have a singlet spin state. They tend to exist in an equilibrium between monomeric and dimeric forms in solution. In contrast carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s sometimes have a triplet ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state ...
and in all cases readily dimerize.
In dimethyllead, (CH3)2Pb, the Pb–C bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
is 2.267 Å and the C–Pb–C bond angle
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that deter ...
is 93.02°; the singlet–triplet gap is 36.99 kcal mol−1.
 The Pb–C bond distance was found to be 2.303 Å and the C–Pb–C angle 105.7°. Notwithstanding the different levels of theory, the larger bond angle for (C6H5)2Pb compared to can be rationalized by steric effects.
Plumbylenes occur as reactive intermediates in the formation of tetravalent
The Pb–C bond distance was found to be 2.303 Å and the C–Pb–C angle 105.7°. Notwithstanding the different levels of theory, the larger bond angle for (C6H5)2Pb compared to can be rationalized by steric effects.
Plumbylenes occur as reactive intermediates in the formation of tetravalent plumbane
Plumbane is an inorganic chemical compound with the chemical formula PbH. It is a colorless gas. It is a metal hydride and group 14 hydride composed of lead and hydrogen. Plumbane is not well characterized or well known, and it is thermodynamicall ...
s (R4Pb). Although the inert pair effect suggests the divalent state should be thermodynamically more stable than the tetravalent state, in the absence of stabilizing substituents, plumbylenes are sensitive to heat and light, and tend to undergo polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
and disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
, forming elemental lead in the process.
Plumbylenes can be stabilized as monomers by the use of sterically bulky ligands (kinetic stabilization) or heteroatom-containing substituents that can donate electron density into the vacant 6p orbital (thermodynamic stabilization).
Dimerization
 Organoplumbylenes tend to exist in an equilibrium between the monomeric and dimeric form in solution, and, due to the low dimerization energy, as either monomers or dimers in the solid state, depending on the steric bulk of substituents. Bulky substituents allow the plumbylene to exist exclusively as monomers. In the dimerization, a
Organoplumbylenes tend to exist in an equilibrium between the monomeric and dimeric form in solution, and, due to the low dimerization energy, as either monomers or dimers in the solid state, depending on the steric bulk of substituents. Bulky substituents allow the plumbylene to exist exclusively as monomers. In the dimerization, a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
ic vacant 6p orbital interacts with a weakly Lewis basic 6s lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
. These diplumbenes possess a ''trans''-bent structure similar to that in lighter, non-carbon congeners (
These diplumbenes possess a ''trans''-bent structure similar to that in lighter, non-carbon congeners (disilene
Disilene is an inorganic compound with the chemical formula . The name ''disilene'', referring to the structure of a particular prototropic tautomer of the molecule. It is the simplest silenes, silene.
Properties and bonding
Disilene is a molec ...
s, digermylenes, distannylenes). The observed Pb–Pb bond lengths in diplumbenes (2.90 – 3.53 Å) have been found to typically be longer than those in tetravalent diplumbanes R3PbPbR3 (2.84 – 2.97 Å). This, together with the low computed dimerization energy (energy released from the formation of dimers from monomers) of 24 kJ mol−1 for Pb2H4, indicates weak multiple bond
Multiple may refer to:
Economics
*Multiple finance, a method used to analyze stock prices
*Multiples of the price-to-earnings ratio
*Chain stores, are also referred to as 'Multiples'
*Box office multiple, the ratio of a film's total gross to tha ...
ing. This counterintuitive result is due to the pair of 6s-6p donor-acceptor interactions representing the Pb=Pb double bond in diplumbenes being less energetically favourable compared to the overlap of spn orbitals (with a higher degree of hybridization than in diplumbenes) in the Pb–Pb single bond in diplumbanes.
Monohalogenated plumbylenes dimerize by formation of bridging halide. The halogen donate a lone pair into the vacant 6p orbital of a second lead atom. Again, sufficiently bulky substituents on lead can sterically block this dimerization mode.
Due to decreasing dimerization energy down Group 14, while monohalogenated stannylenes and plumbylenes dimerize via the halogen-bridging mode, monohalogenated silylenes and germylenes tend to dimerize ''via'' the abovementioned multiply-bonded mode instead. ''N''-heterocyclic plumbylene also dimerize leading to C–H activation, existing in solution in an equilibrium between the monomer and a dimer resulting from cleavage of an aryl C–H bond and formation of Pb–C and N–H bonds.
''N''-heterocyclic plumbylene also dimerize leading to C–H activation, existing in solution in an equilibrium between the monomer and a dimer resulting from cleavage of an aryl C–H bond and formation of Pb–C and N–H bonds.
Stabilizing intramolecular interactions with substituents bearing lone pairs
Plumbylenes may be stabilized by electron donation into the vacant orbital of the lead atom. The two common intramolecular modes are resonance from a lone pair on the atom directly attached to the lead or by coordination from aLewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
elsewhere in the molecule.
For example, Group 15 or 16 elements directly adjacent to Pb donate a lone pair in manner similar to their stabilizing effect on Fisher carbenes. Common examples of more remote electron-donors include nitrogen atoms that can lead to a six-memberd ring by bonding to the lead. Even a fluorine atom on a remote trifluoromethyl
The trifluoromethyl group is a functional group that has the formula . The naming of is group is derived from the methyl group (which has the formula ), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane ...
group has been seen forming a coordination to lead in ,4,6-(CF3)3C6H2sub>2Pb.
Agostic interactions
Agostic interactions have also been shown to stabilize plumbylenes. DFT computations on the compounds R(CH3)2Si)CHsub>2Pb (R = Me or Ph) found that agostic interactions between bonding B–H orbitals and the vacant 6p orbital lowered the energy of the molecule by ''ca.'' 38 kcal mol−1; this was supported by X-ray crystal structures showing the favourable positioning of said B–H bonds in proximity of Pb.Reactivity
As previously mentioned, unstabilized plumbylenes are prone to polymerization and disproportionation, and plumbylenes without bulky substituents tend to dimerize in one of two modes. Below, the reactions of stabilized plumbylenes (at least at the temperatures at which they were studied) are listed.Lewis acid-base adduct formation
Plumbylenes are Lewis acidic ''via'' the vacant 6p orbital and tend to form adducts with Lewis bases, such as trimethylamine ''N''-oxide (Me3NO), 1-azidoadamantane (AdN3), andmesityl
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzen ...
azide (MesN3). In contrast, the reaction between stannylenes and Me3NO produces the corresponding distannoxane (from oxidation of Sn(II) to Sn(IV)) instead of the Lewis adduct, which can be attributed to tin being a period above Pb, experiencing the inert pair effect to a lesser degree and hence having a higher susceptibility to oxidation.
In reactions with azides, the precise nature of adducts depends on the moieties near the lead(II) center. A nearby phosphorus atom also acts as a Lewis base, and in the known instance forms a bridging ring; absent such an atom, the azide evolves N2 to form a nitrene, which then inserts into a C-H bond of an arene substituent and coordinates to Pb as an amine base.

Insertion
Similar to carbenes and other Group 14 congeners, plumbylenes have been shown to undergo insertion reactions, specifically into C–X (X = Br, I) and Group 16 E–E (E = S, Se) bonds. Insertions into lead-substituent bonds can also occur.27 In the examples below, insertion is accompanied by intramolecular rearrangement to place more electron-donating heteroatoms next to the electron-deficient lead.27
Insertions into lead-substituent bonds can also occur.27 In the examples below, insertion is accompanied by intramolecular rearrangement to place more electron-donating heteroatoms next to the electron-deficient lead.27
Transmetallation
Plumbylenes are known to undergo nucleophilic substitution with organometallic reagents to form transmetallated products.28 In an unusual example, the use of TlPF6, bearing theweakly coordinating anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found ...
PF6−, led to the formation of crystals of an oligonuclear lead compound with a chain structure upon work-up, highlighting the interesting reactivity of plumbylenes.28
In addition, plumbylenes can also undergo metathesis with group 13
The Group 13 network (, ) was a Jewish collaborationist organization in the Warsaw Ghetto during the German occupation of Poland in World War II. The rise and fall of the Group was likely a proxy for power struggles between various facti ...
E(CH3)3 (E = Al, Ga) compounds.
Plumbylenes bearing different substituents can also undergo transmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
and exchange substituents, with the driving force being the relief of steric strain Van der Waals strain is strain resulting from Van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their Van der Waals radii.
Van der Waals strain is also called Van der Waals repul ...
and the low Pb-C bond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
.

Applications
Plumbylenes can be used as concurrent σ-donor-σ-acceptorligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s to metal complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or ...
es, functioning as σ-donor via its filled 6s orbital and σ-acceptor via its empty 6p orbital.
Room temperature-stable plumbylenes have also been suggested as precursors in chemical vapour deposition (CVD) and atomic layer deposition (ALD) of lead-containing materials. Dithioplumbylenes and dialkoxyplumbylenes may be useful as precursors for preparing the semiconductor material lead sulphide and piezoelectric PZT respectively.
References
{{reflist Octet-deficient functional groups Organolead compounds