Phosphorus Mononitride on:
[Wikipedia]
[Google]
[Amazon]
Phosphorus mononitride is an
 PN formation from gaseous phosphorus and nitrogen is
PN formation from gaseous phosphorus and nitrogen is  Its dipole moment is larger than PO (1.88 D), despite the greater
Its dipole moment is larger than PO (1.88 D), despite the greater
 Monomeric PN can only be isolated in krypton or argon matrices at . Upon warming up past , cyclotriphosphazene, which has D3h symmetry, is formed (up to before krypton matrix melts). The (PN)3 trimer and is planar and aromatic, with 15N-labelling experiments revealing a planar E' mode band at 1141 cm−1. No dimers or other oligomers are even transiently observed.
Without a cryoscopic matrix, these reactions result in the immediate formation of (PN)n polymers.
Thermolysis experiments of dimethyl phosphoramidate have shown PN to form as a major decomposition product along with many other minor components including the ·P=O radical and HOP=O. This is contrasting to
Monomeric PN can only be isolated in krypton or argon matrices at . Upon warming up past , cyclotriphosphazene, which has D3h symmetry, is formed (up to before krypton matrix melts). The (PN)3 trimer and is planar and aromatic, with 15N-labelling experiments revealing a planar E' mode band at 1141 cm−1. No dimers or other oligomers are even transiently observed.
Without a cryoscopic matrix, these reactions result in the immediate formation of (PN)n polymers.
Thermolysis experiments of dimethyl phosphoramidate have shown PN to form as a major decomposition product along with many other minor components including the ·P=O radical and HOP=O. This is contrasting to  In 2023, Qian et al. proposed PN to be generated as a major product along with CO and cyclopentadienone byproducts when (o-phenyldioxyl)phosphinoazide is heated to 850 °C (following the loss of N2). However, efforts to observe free PN in argon matrices using this method were unsuccessful due to band overlaps.
In 2023, Qian et al. proposed PN to be generated as a major product along with CO and cyclopentadienone byproducts when (o-phenyldioxyl)phosphinoazide is heated to 850 °C (following the loss of N2). However, efforts to observe free PN in argon matrices using this method were unsuccessful due to band overlaps.
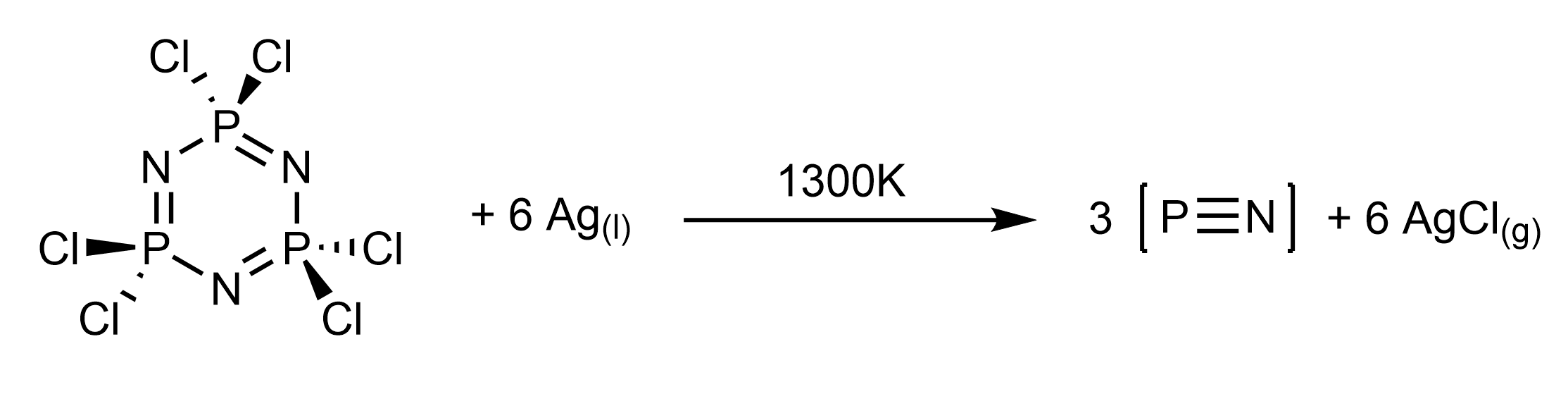 Schnöckel and coworkers later showed an alternative synthesis involving the dehalogenation of
Schnöckel and coworkers later showed an alternative synthesis involving the dehalogenation of
 The aforementioned methods require very high temperatures which are incompatible with standard, homogeneous solution state chemistry.
In 2022,
The aforementioned methods require very high temperatures which are incompatible with standard, homogeneous solution state chemistry.
In 2022,
 Phosphorus mononitride's tendency to rapidly polymerize with itself has dominated its reactivity, greatly hindering both the study and diversity of products in its reactions with organic molecules.
In 2023, a rare case of documented reactivity with an organic molecule was reported by Qian and coworkers who demonstrated reversible photoisomerization between ''o''-benzoquinone supported phosphinonitrene and ''o''-benzoquinone stabilized phosphorus mononitride at 10 K, which can be isolated in an argon matrix.
Phosphorus mononitride's tendency to rapidly polymerize with itself has dominated its reactivity, greatly hindering both the study and diversity of products in its reactions with organic molecules.
In 2023, a rare case of documented reactivity with an organic molecule was reported by Qian and coworkers who demonstrated reversible photoisomerization between ''o''-benzoquinone supported phosphinonitrene and ''o''-benzoquinone stabilized phosphorus mononitride at 10 K, which can be isolated in an argon matrix.
 Smith and co-workers isolated the first stable M-PN (and M-NP) complexes, using methodology to generate the PN moiety at metal sites. They reacted a tris(amido) Mo(VI) terminal phosphide complex with a tris(carbene)borate Fe(IV) terminal nitride, which undergo reductive coupling to form the corresponding neutral bridging PhB(iPr2Im)3Fe-NP-Mo(N3N) complex. Notably, the Mo-N-P bond angle in the bridging compound is nearly perfectly linear with an N-P bond length of 1.509(6) Å (only slightly elongated from free PN indicating significant multiple bond character). Addition of 3 equivalents of strongly lewis basic tert-butyl
Smith and co-workers isolated the first stable M-PN (and M-NP) complexes, using methodology to generate the PN moiety at metal sites. They reacted a tris(amido) Mo(VI) terminal phosphide complex with a tris(carbene)borate Fe(IV) terminal nitride, which undergo reductive coupling to form the corresponding neutral bridging PhB(iPr2Im)3Fe-NP-Mo(N3N) complex. Notably, the Mo-N-P bond angle in the bridging compound is nearly perfectly linear with an N-P bond length of 1.509(6) Å (only slightly elongated from free PN indicating significant multiple bond character). Addition of 3 equivalents of strongly lewis basic tert-butyl  Cummins and co-workers exploited their N3PA free PN releasing reagent to "trap" and isolate a stable terminal (dppe)(Cp*)Fe-NP complex as a BArF24 salt. The NP bond length in this case was very short at 1.493(2) Å, almost unperturbed from gaseous PN, which is consistent with minimal pi-backbonding from the iron center. Studies confirmed the NP binding mode (as opposed to PN) to be energetically preferred by in this iron complex, creating a significant barrier to isomerization (thought to arise from
Cummins and co-workers exploited their N3PA free PN releasing reagent to "trap" and isolate a stable terminal (dppe)(Cp*)Fe-NP complex as a BArF24 salt. The NP bond length in this case was very short at 1.493(2) Å, almost unperturbed from gaseous PN, which is consistent with minimal pi-backbonding from the iron center. Studies confirmed the NP binding mode (as opposed to PN) to be energetically preferred by in this iron complex, creating a significant barrier to isomerization (thought to arise from  The V-NP fragment undergoes singlet phosphinidene reactivity ( +1additions) with alkene and alkyne trapping agents, generating phosphiranes and phospherenes respectively. The products generated from such additions exist in equilibrium (in the case with cis-4-octene and bis-trimethylsilylacetylene), where retention of the cis-4-octene conformer is observed. Upon heating, they reversibly add to generate the V-NP dimer. Such reactivity demonstrates stark contrasts from P2 as a ligand which instead undergoes formal
The V-NP fragment undergoes singlet phosphinidene reactivity ( +1additions) with alkene and alkyne trapping agents, generating phosphiranes and phospherenes respectively. The products generated from such additions exist in equilibrium (in the case with cis-4-octene and bis-trimethylsilylacetylene), where retention of the cis-4-octene conformer is observed. Upon heating, they reversibly add to generate the V-NP dimer. Such reactivity demonstrates stark contrasts from P2 as a ligand which instead undergoes formal 
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
. Containing only phosphorus and nitrogen, this material is classified as a binary
Binary may refer to:
Science and technology Mathematics
* Binary number, a representation of numbers using only two values (0 and 1) for each digit
* Binary function, a function that takes two arguments
* Binary operation, a mathematical op ...
nitride. From the Lewis structure perspective, it can be represented with a P-N triple bond with a lone pair on each atom. It is isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
with , CO, , CS, , and SiO.
The compound is highly unstable in standard conditions, tending to rapidly self polymerize. It can be isolated within argon and krypton matrices at . Due to its instability, documentation of reactions with other molecules is limited. Most of its reactivity has thus far been probed and studied at transition metal centers.
Phosphorus mononitride was the first identified phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
compound in the interstellar medium and is even thought to be an important molecule in the atmospheres of Jupiter and Saturn.
Discovery and interstellar occurrence
The existence of free, gas-phase phosphorus mononitride was confirmed spectroscopically in 1934 by Nobel laureate,Gerhard Herzberg
Gerhard Heinrich Friedrich Otto Julius Herzberg, (; December 25, 1904 – March 3, 1999) was a German-Canadian pioneering physicist and physical chemist, who won the Nobel Prize for Chemistry in 1971, "for his contributions to the knowledge ...
, and coworkers. J. Curry, L. Herzberg, and G. Herzberg made the accidental discovery after observing new bands in the UV region from 2375 to 2992 Å following an electric discharge within an air-filled tube that had been earlier exposed to phosphorus.
In 1987, phosphorus mononitride was detected in the Orion KL Nebula, the W51M nebula in Aquila
Aquila may refer to:
Arts, entertainment, and media
* ''Aquila'', a series of books by S.P. Somtow
* ''Aquila'', a 1997 book by Andrew Norriss
* ''Aquila'' (children's magazine), a UK-based children's magazine
* ''Aquila'' (journal), an orni ...
, and Saggitarius B2 simultaneously by Turner, Bally, and Ziurys. Data from radio telescopes allowed for observation of rotational lines associated with the J = 2-1, 3-2, 5-4, and 6-5 transitions.
In the following decades, a rapid expansion of interstellar PN observations ensued, detected frequently alongside PO. Examples include within shocked regions of L1157, within the Galactic Center
The Galactic Center is the barycenter of the Milky Way and a corresponding point on the rotational axis of the galaxy. Its central massive object is a supermassive black hole of about 4 million solar masses, which is called Sagittarius A*, a ...
, in carbon-rich envelopes in CRL 2688
The Egg Nebula (also known as RAFGL 2688 and CRL 2688) is a bipolar nebula, bipolar protoplanetary nebula approximately 3,000 light-years away from Earth. Its peculiar properties were first described in 1975 using data from the 11 μm survey ...
(alongside HCP) and oxygen-rich envelopes toward VY Canis Majoris
VY Canis Majoris (abbreviated to VY CMa) is an extreme oxygen-rich red hypergiant or red supergiant (O-rich RHG or RSG) and pulsating variable star from the Solar System in the slightly southern constellation of Canis Major. It is on ...
, TX Camelopardalis, R. Cassiopeiae, and NML Cygni
NML Cygni or V1489 Cygni (abbreviated to NML Cyg or V1489 Cyg) is a M-type star, red hypergiant or red supergiant (RSG) in the constellation Cygnus (constellation), Cygnus. It is possibly one of the List of largest stars, largest known ...
.
ALMA
Alma or ALMA may refer to:
Arts and entertainment
* ''Alma'' (film), a 2009 Spanish short animated film
* ''Alma'', an upcoming film by Sally Potter
* ''Alma'' (Oswald de Andrade novel), 1922
* ''Alma'' (Le Clézio novel), 2017
* ''Alma'' ( ...
data alongside spectroscopic measurements from the Rosetta
Rosetta ( ) or Rashid (, ; ) is a port city of the Nile Delta, east of Alexandria, in Egypt's Beheira governorate. The Rosetta Stone was discovered there in 1799.
Founded around the 9th century on the site of the ancient town of Bolbitine, R ...
probe have shown PN being carried from the comet 67P/Churyumov–Gerasimenko
67P/Churyumov–Gerasimenko (abbreviated as 67P or 67P/C–G) is a Jupiter-family comet. It is originally from the Kuiper belt and has an orbital period of 6.45 years as of 2012, a rotation period of approximately 12.4 hours, and a maximum velo ...
alongside the far more abundant PO. These observations may offer insight to how pre-biotic matter could be transported to planets. In cases where PN and PO are observed in the same region, the latter is more abundant. The consistency of the molecular ratio between these two interstellar molecules across many different interstellar cloud
An interstellar cloud is an accumulation of gas, plasma, and cosmic dust in galaxies. Put differently, an interstellar cloud is a denser-than-average region of the interstellar medium, the matter and radiation that exists in the space between ...
s is thought to be a sign of a shared formation pathway between the two molecules. PN is mostly detected in hot, turbulent regions, where the shock induced sputtering of dust grain is thought to contribute to its formation. However, it has also been confirmed in massive dense cores which are by comparison "cold and quiescent".
In 2022, researchers used data from the ALMA Comprehensive High-resolution Extragalactic Molecular Inventory (ALCHEMI) project and reported evidence of phosphorus mononitride in giant molecular clouds within the galaxy, NGC 253
The Sculptor Galaxy (also known as the Silver Coin Galaxy, Silver Dollar Galaxy, NGC 253, or Caldwell 65) is an intermediate spiral galaxy in the constellation Sculptor. The Sculptor Galaxy is a starburst galaxy, which means that it is currently ...
. This finding marks phosphorus mononitride as the first extragalactic phosphorus containing molecule detected as well. In 2023, Ziurys and coworkers showed the existence of PN and PO in WB89-621 (22.6 kpc from the Galactic Center
The Galactic Center is the barycenter of the Milky Way and a corresponding point on the rotational axis of the galaxy. Its central massive object is a supermassive black hole of about 4 million solar masses, which is called Sagittarius A*, a ...
) using rotational spectroscopy. Prior, phosphorus was only observed in the inner Milky Way (12kpc). Since supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion ...
e do not occur in outer regions of the galaxy, the detection of these phosphorus-bearing molecules in WB89-621 provides evidence of additional alternative sources of phosphorus formation, such as non-explosive, lower mass asymptotic giant branch stars. The levels were detected at comparable values to that in the Solar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
.
Electronic structure, spectral and bonding properties
 PN formation from gaseous phosphorus and nitrogen is
PN formation from gaseous phosphorus and nitrogen is endothermic
An endothermic process is a chemical or physical process that absorbs heat from its surroundings. In terms of thermodynamics, it is a thermodynamic process with an increase in the enthalpy (or internal energy ) of the system.Oxtoby, D. W; Gillis, ...
.
½ P2 + ½ N2 = PN (ER = 117 ± 10kJ/mol)
Early mass spectrometry studies by Gingerich yielded a PN dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
D0 of .
It is predicted to have a high proton affinity
The proton affinity (PA, ''E''pa) of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase:
::: A- + H+ -> HA
::: B + H+ -> BH+ ...
(PA = ).
Early rotational analysis of 24 of the bands from Herzberg's original study suggested a PN internuclear distance of 1.49 Å, intermediate between N2 (1.094 Å) and P2 (1.856 Å). The associated electronic transition, 1Π → 1Σ, was noted to be similar to that of the isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in th ...
CS and SiO molecules. Later rotational spectra studies aligned well with these findings, for example analysis of millimeter wave rotational PN spectra from a microwave spectrometer yielded a bond distance of 1.49085 (2) Å.
Infrared studies of gaseous PN at high temperatures assign its vibrational frequency (ωe) to 1337.24 cm−1 and interatomic separation of 1.4869 Å.
Simple comparisons to tabulated experimental and calculated bond lengths match well with a PN triple bond according to Pyykkö's Triple-Bond Covalent Radii.
NBO analyses support a single neutral resonance structure
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
with a PN triple bond and one lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
on each atom. However, natural population analysis shows nitrogen as significantly negatively charged (-0.82603) and phosphorus as significantly positively charged (0.82603). This is in line with the large dipole moment and partial ionic character reflecting the electron density contour plots.
Monomeric PN in a krypton matrix at gives rise to a single IR band at 1323 cm−1.
Auer and Neese have produced calculated gas phase 31P and 15N NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which atomic nucleus, nuclei in a strong constant magnetic field are disturbed by a weak oscillating magnetic field (in the near and far field, near field) and respond by producing ...
chemical shifts of 51.61 and -344.71 respectively at the CCSD(T)/p4 level of theory. However, different functionals and basis sets yield dramatically different predictions for chemical shielding and so far experimental NMR shifts for phosphorus mononitride remain elusive.
Molecular beam electric resonance spectroscopy has been used to determine the radio frequency spectrum of phosphorus mononitride generated from P3N5 thermolysis; the experimental results showed an experimental PN dipole moment (μ) of 2.7465 +- 0.0001 D, 2.7380 +-0.001 D, and 2.7293 +-0.0001 D for the first three vibrational levels respectively.
 Its dipole moment is larger than PO (1.88 D), despite the greater
Its dipole moment is larger than PO (1.88 D), despite the greater electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
difference between the constituent P and O atoms and similar bond length (1.476 Å). This a result of the significant differences in bonds and charge distribution within the PN and PO molecules. The large PN dipole moment makes it very favorable with respect to radio-astronomical studies in comparison to N2 - which lacks this property.
In consideration to molecular orbitals of PN, direct analogies can be drawn to the bonding in the N2 molecule. It consists of an P-N σ bonding orbital (HOMO), with two perpendicular degenerate P-N pi bonding orbitals. Likewise, the LUMOs of PN, which consist of a degenerate PN pi-antibonding set, allow it to backbond with orbitals of appropriate symmetry.
However, in comparison to N2, the HOMO of PN is higher in energy (est. -9.2 eV vs -12.2 eV), and, the LUMOs are lower in energy (-2.3 eV vs -0.6 eV), thus making it both a better σ-donor and pi-acceptor as a ligand.
Evidently, the smaller HOMO-LUMO gap of PN, combined with its polar nature and low dissociation energy contribute to its much greater reactivity than dinitrogen (including at the interstellar level).
Preparation and formation
Interstellar formation
The pathways to the formation of PN are still not fully understood, but likely involve competing gaseous phase reactions with other interstellar molecules. Important schemes are shown below along with competing exothermic reactions: PO + N → PN + O PO + N → P + NO (Competing) Another important, very exothermic formation reaction: PH + N → PN + H From carbon containing environments: P + CN → PN + C N + CP → PN + C An important destruction pathway: PN + N → N2 + P The abundance of interstellar PN is additionally perturbed by cosmic-ray ionization, visual extinction, and adsorption/desorption from dust grains.Electric discharge
Moldenhauer and Dörsam first generated transient PN in 1924 using an electric discharge through N2 andphosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
vapors, where the characterized product was a notably robust powder containing equal parts phosphorus and nitrogen. This same method led to the actual first observation of PN by Gerhard and coworkers.
PN has also been produced at room temperature using microwave discharges on mixtures of gaseous PCl3 and N2 under moderate vacuum. This preparation was employed to achieve high resolution FTIR spectra of PN.
Flash pyrolysis
Atkins and Timms later generated PN via flashpyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
of P3N5 under high vacuum, allowing the recording of the PN infrared spectrum within a cryogenic krypton matrix
Matrix (: matrices or matrixes) or MATRIX may refer to:
Science and mathematics
* Matrix (mathematics), a rectangular array of numbers, symbols or expressions
* Matrix (logic), part of a formula in prenex normal form
* Matrix (biology), the m ...
. Solid triphosphorus pentanitride generates gaseous, free PN when heated to under high vacuum.
 Monomeric PN can only be isolated in krypton or argon matrices at . Upon warming up past , cyclotriphosphazene, which has D3h symmetry, is formed (up to before krypton matrix melts). The (PN)3 trimer and is planar and aromatic, with 15N-labelling experiments revealing a planar E' mode band at 1141 cm−1. No dimers or other oligomers are even transiently observed.
Without a cryoscopic matrix, these reactions result in the immediate formation of (PN)n polymers.
Thermolysis experiments of dimethyl phosphoramidate have shown PN to form as a major decomposition product along with many other minor components including the ·P=O radical and HOP=O. This is contrasting to
Monomeric PN can only be isolated in krypton or argon matrices at . Upon warming up past , cyclotriphosphazene, which has D3h symmetry, is formed (up to before krypton matrix melts). The (PN)3 trimer and is planar and aromatic, with 15N-labelling experiments revealing a planar E' mode band at 1141 cm−1. No dimers or other oligomers are even transiently observed.
Without a cryoscopic matrix, these reactions result in the immediate formation of (PN)n polymers.
Thermolysis experiments of dimethyl phosphoramidate have shown PN to form as a major decomposition product along with many other minor components including the ·P=O radical and HOP=O. This is contrasting to dimethyl methylphosphonate
Dimethyl methylphosphonate is an organophosphorus compound with the chemical formula CH3PO(OCH3)2. It is a colourless liquid, which is primarily used as a flame retardant.
Synthesis
Dimethyl methylphosphonate can be prepared from trimethyl phosph ...
in which said minor components become the major decomposition products, highlighting significantly diverging pathways.
 In 2023, Qian et al. proposed PN to be generated as a major product along with CO and cyclopentadienone byproducts when (o-phenyldioxyl)phosphinoazide is heated to 850 °C (following the loss of N2). However, efforts to observe free PN in argon matrices using this method were unsuccessful due to band overlaps.
In 2023, Qian et al. proposed PN to be generated as a major product along with CO and cyclopentadienone byproducts when (o-phenyldioxyl)phosphinoazide is heated to 850 °C (following the loss of N2). However, efforts to observe free PN in argon matrices using this method were unsuccessful due to band overlaps.
Dehalogenation of hexachlorophosphazene
 Schnöckel and coworkers later showed an alternative synthesis involving the dehalogenation of
Schnöckel and coworkers later showed an alternative synthesis involving the dehalogenation of hexachlorophosphazene
Hexachlorophosphazene is an inorganic compound with the chemical formula . The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen atoms, and can be viewed as a trimer of the hypothetical compound (p ...
with molten silver, with concomitant loss of AgCl. In both this route and the P3N5 thermolysis route, only trace P2 and P4 formation is detected even at , showing the reaction temperatures occur far from thermodynamic equilibrium.
Anthracene release from dibenzo-7λ3 -phosphanorbornadiene derivatives
 The aforementioned methods require very high temperatures which are incompatible with standard, homogeneous solution state chemistry.
In 2022,
The aforementioned methods require very high temperatures which are incompatible with standard, homogeneous solution state chemistry.
In 2022, Cummins
Cummins Inc. is an American multinational corporation, multinational corporation that designs, manufactures, and distributes engines, electric vehicle components, and power generation products. Cummins also services engines and related equipmen ...
and coworkers prepared and isolated a molecular PN precursor, N3PA which rapidly decomposes to N2, anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes, as a scintil ...
, and PN in solution at room temperature (t½ = 30 minutes). With the combination of vacuum and heating to 42 °C, this dissociation is explosive.
Reactivity
Reactions of phosphorus mononitride with other molecules are rare and rather difficult to carry out. The formation of the intermediate (PN)3 trimer (which itself is only isolated in matrices) is highly favorable: 3PN ⇌ (PN)3 (-334 +/- 60 kJ/mol) PN generated in both the gaseous phase or in solution that is not subjected to trapping via noble gas matrices or particular metal complexes results in rapid self polymerization even in cases where trapping agents such as dienes or alkynes are present (differentiating its reactivity profile from related molecules such as P2). Phosphorus mononitride's tendency to rapidly polymerize with itself has dominated its reactivity, greatly hindering both the study and diversity of products in its reactions with organic molecules.
In 2023, a rare case of documented reactivity with an organic molecule was reported by Qian and coworkers who demonstrated reversible photoisomerization between ''o''-benzoquinone supported phosphinonitrene and ''o''-benzoquinone stabilized phosphorus mononitride at 10 K, which can be isolated in an argon matrix.
Phosphorus mononitride's tendency to rapidly polymerize with itself has dominated its reactivity, greatly hindering both the study and diversity of products in its reactions with organic molecules.
In 2023, a rare case of documented reactivity with an organic molecule was reported by Qian and coworkers who demonstrated reversible photoisomerization between ''o''-benzoquinone supported phosphinonitrene and ''o''-benzoquinone stabilized phosphorus mononitride at 10 K, which can be isolated in an argon matrix.
Ligation, stabilization, and reactivity at transition metals
The majority of documented well-defined PN reactivity has been carried out at transition metal centers. The electronic and molecular orbital similarities it shares with N2 make it a viable ligating species. While free PN is unstable, phosphorus mononitride has been prepared at metal coordination sites where it can exist as an isolable terminal ligand within a complex. In alternative cases, PN ligands can also exist as only as transient, highly reactive intermediates featuring rich chemistry. As a terminal ligand, cases of both preferential P and N bonding modes have been discovered. Smith and co-workers isolated the first stable M-PN (and M-NP) complexes, using methodology to generate the PN moiety at metal sites. They reacted a tris(amido) Mo(VI) terminal phosphide complex with a tris(carbene)borate Fe(IV) terminal nitride, which undergo reductive coupling to form the corresponding neutral bridging PhB(iPr2Im)3Fe-NP-Mo(N3N) complex. Notably, the Mo-N-P bond angle in the bridging compound is nearly perfectly linear with an N-P bond length of 1.509(6) Å (only slightly elongated from free PN indicating significant multiple bond character). Addition of 3 equivalents of strongly lewis basic tert-butyl
Smith and co-workers isolated the first stable M-PN (and M-NP) complexes, using methodology to generate the PN moiety at metal sites. They reacted a tris(amido) Mo(VI) terminal phosphide complex with a tris(carbene)borate Fe(IV) terminal nitride, which undergo reductive coupling to form the corresponding neutral bridging PhB(iPr2Im)3Fe-NP-Mo(N3N) complex. Notably, the Mo-N-P bond angle in the bridging compound is nearly perfectly linear with an N-P bond length of 1.509(6) Å (only slightly elongated from free PN indicating significant multiple bond character). Addition of 3 equivalents of strongly lewis basic tert-butyl isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragme ...
results in the release of the iron adduct as a hB(iPr2Im)3Fe-(CNtBu)3sup>+ cation in the second coordination sphere. The corresponding terminal linear Mo-PN anion can be isolated and converted to its linear Mo-NP isomer by exposure to white light in the solid state. The M-NP isomer of the ligand was determined to be more pi-acidic (N-P = 1.5913(1) Å and P-N = 1.5363(1) Å) and more thermodynamically stable than its isomer.
 Cummins and co-workers exploited their N3PA free PN releasing reagent to "trap" and isolate a stable terminal (dppe)(Cp*)Fe-NP complex as a BArF24 salt. The NP bond length in this case was very short at 1.493(2) Å, almost unperturbed from gaseous PN, which is consistent with minimal pi-backbonding from the iron center. Studies confirmed the NP binding mode (as opposed to PN) to be energetically preferred by in this iron complex, creating a significant barrier to isomerization (thought to arise from
Cummins and co-workers exploited their N3PA free PN releasing reagent to "trap" and isolate a stable terminal (dppe)(Cp*)Fe-NP complex as a BArF24 salt. The NP bond length in this case was very short at 1.493(2) Å, almost unperturbed from gaseous PN, which is consistent with minimal pi-backbonding from the iron center. Studies confirmed the NP binding mode (as opposed to PN) to be energetically preferred by in this iron complex, creating a significant barrier to isomerization (thought to arise from Pauli repulsion
In chemistry and physics, the exchange interaction is a quantum mechanical constraint on the states of indistinguishable particles. While sometimes called an exchange force, or, in the case of fermions, Pauli repulsion, its consequences cannot alw ...
effects). Studies of phosphorus mononitride chemistry at tris(amido) vanadium complexes undertaken by Cummins and coworkers provides the bulk of PN reactivity examples at transition metals to date. In this system, PN is synthetically generated at a vanadium center from respective dibenzo-7λ3 -phosphanorbornadiene derivative precursors. However, it is not stable as a terminal ligand, and instead immediately undergoes trimerization. Notably, a thermodynamic equilibrium exists between this trimer species, along with a dimer and non-observed monomeric intermediate fragment.
 The V-NP fragment undergoes singlet phosphinidene reactivity ( +1additions) with alkene and alkyne trapping agents, generating phosphiranes and phospherenes respectively. The products generated from such additions exist in equilibrium (in the case with cis-4-octene and bis-trimethylsilylacetylene), where retention of the cis-4-octene conformer is observed. Upon heating, they reversibly add to generate the V-NP dimer. Such reactivity demonstrates stark contrasts from P2 as a ligand which instead undergoes formal
The V-NP fragment undergoes singlet phosphinidene reactivity ( +1additions) with alkene and alkyne trapping agents, generating phosphiranes and phospherenes respectively. The products generated from such additions exist in equilibrium (in the case with cis-4-octene and bis-trimethylsilylacetylene), where retention of the cis-4-octene conformer is observed. Upon heating, they reversibly add to generate the V-NP dimer. Such reactivity demonstrates stark contrasts from P2 as a ligand which instead undergoes formal cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
chemistry.

Applications
The robust nature of PN reaction products such as (PN)n, could find use in heat resistantceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcela ...
s or as fire suppressing materials.
There has long been interest in studying PN and its reaction products like (PN)n polymers, noting their relevance to precursors/intermediates in the production of fertilizer
A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Man ...
s.
See also
* Triphosphorus pentanitride *Phosphorus monoxide
Phosphorus monoxide is an unstable radical inorganic compound with molecular formula P O.
Phosphorus monoxide is notable as one of the few molecular compounds containing phosphorus that has been detected outside of Earth. Other phosphorus cont ...
* Diphosphorus
Diphosphorus is an inorganic chemical with the chemical formula . Unlike nitrogen, its lighter pnictogen neighbor which forms a stable N2 molecule with a nitrogen to nitrogen triple bond, phosphorus prefers a tetrahedral form P4 because P-P pi-bo ...
* Carbon monosulfide
Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interst ...
* Silicon monoxide
Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule.
It has been detected in stellar objects and has been described as the most common o ...
References
{{Nitrides Phosphorus-nitrogen compounds Solids