Phorbol Synthesis on:
[Wikipedia]
[Google]
[Amazon]
Phorbol is a natural, plant-derived
 The main results of phorbol exposure are tumor promotion and inflammatory response. Although phorbol is not a carcinogen itself, it greatly enhances the action of other substances and promotes tumor proliferation. PKC is a key component in biological pathways controlling cell growth and differentiation. When phorbol esters bind to PKC, cell proliferation pathways are activated. This effect greatly promotes tumors when the cells are exposed to even a sub-carcinogenic amount of a substance.
PKC is also involved in activation of inflammation pathways such as the
The main results of phorbol exposure are tumor promotion and inflammatory response. Although phorbol is not a carcinogen itself, it greatly enhances the action of other substances and promotes tumor proliferation. PKC is a key component in biological pathways controlling cell growth and differentiation. When phorbol esters bind to PKC, cell proliferation pathways are activated. This effect greatly promotes tumors when the cells are exposed to even a sub-carcinogenic amount of a substance.
PKC is also involved in activation of inflammation pathways such as the
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
. It is a member of the tigliane
Tigliane is a diterpene
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyro ...
family of diterpenes
Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a prima ...
. Phorbol was first isolated in 1934 via the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of croton oil
Croton oil (''Crotonis oleum'') is an oil prepared from the seeds of '' Croton tiglium'', a tree belonging to the order Euphorbiales and family Euphorbiaceae, and native or cultivated in India and the Malay Archipelago. Small doses taken interna ...
, which is derived from the seeds of the purging croton, '' Croton tiglium''. The structure of phorbol was determined in 1967.
Various esters of phorbol have important biological properties, the most notable of which is the capacity to act as tumor promoters through activation of protein kinase C
In cell biology, protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and t ...
. They mimic diacylglycerol
A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are n ...
s, glycerol derivatives in which two hydroxyl groups have reacted with fatty acid
In chemistry, in particular in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an ...
s to form esters. The most common and potent phorbol ester is 12-''O''-tetradecanoylphorbol-13-acetate (TPA), also called phorbol-12-myristate-13-acetate (PMA), which is used as a biomedical research tool in contexts such as models of carcinogenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cell (biology), cells are malignant transformation, transformed into cancer cells. The process is characterized by changes at the cellular, G ...
.
History and source
Phorbol is a natural product found in many plants, especially those of theEuphorbiaceae
Euphorbiaceae (), the spurge family, is a large family of flowering plants. In English, they are also commonly called euphorbias, which is also the name of Euphorbia, the type genus of the family. Most spurges, such as ''Euphorbia paralias'', ar ...
and Thymelaeaceae
The Thymelaeaceae are a cosmopolitan family (biology), family of flowering plants composed of 50 genera (listed below) and 898 species.Zachary S. Rogers (2009 onwards)A World Checklist of Thymelaeaceae (version 1) Missouri Botanical Garden Webs ...
families. Phorbol and phorbol esters are the active constituents of the highly toxic New World tropical manchineel
The manchineel tree (''Hippomane mancinella'') is a species of flowering plant in the spurge family (Euphorbiaceae). Its native range stretches from tropical southern North America to northern South America.
The name ''manchineel'' (sometimes sp ...
tree (''Hippomane mancinella''). It is very soluble in most polar organic solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s, as well as in water. In the manchineel, this leads to an additional exposure risk during rain, where liquid splashing from an undamaged tree may also be injurious. Contact with the tree or consumption of its fruit can lead to symptoms such as severe pain and swelling.
The purging croton, ''Croton tiglium'', is the source of croton oil
Croton oil (''Crotonis oleum'') is an oil prepared from the seeds of '' Croton tiglium'', a tree belonging to the order Euphorbiales and family Euphorbiaceae, and native or cultivated in India and the Malay Archipelago. Small doses taken interna ...
from which phorbol was initially isolated. Its seeds and oil have been used for hundreds of years in traditional medicine, generally as a purgative, and the seeds were mentioned in Chinese herbal texts 2000 years ago. The purgative effects of the oil are largely attributed to the high percentage of phorbol esters contained in the oil. Phorbol was isolated from ''C. tiglium'' seeds in 1934. The structure of the compound was determined in 1967, and a total synthesis was described in 2015..
Mechanism of action
Phorbol derivatives work primarily by interacting with protein kinase C (PKC), although they can interact with other phospholipid membrane receptors. The esters bind to PKC in a similar way to its natural ligand,diacylglycerol
A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are n ...
, and activate the kinase. Diacylglycerol is degraded quickly by the body, allowing PKC to be reversibly activated. When phorbol esters bind to the receptor, they are not degraded as efficiently by the body, leading to constitutively active PK. PKC is involved in a number of important cell signaling pathways. Thus, phorbol ester exposure can show a wide range of results.
 The main results of phorbol exposure are tumor promotion and inflammatory response. Although phorbol is not a carcinogen itself, it greatly enhances the action of other substances and promotes tumor proliferation. PKC is a key component in biological pathways controlling cell growth and differentiation. When phorbol esters bind to PKC, cell proliferation pathways are activated. This effect greatly promotes tumors when the cells are exposed to even a sub-carcinogenic amount of a substance.
PKC is also involved in activation of inflammation pathways such as the
The main results of phorbol exposure are tumor promotion and inflammatory response. Although phorbol is not a carcinogen itself, it greatly enhances the action of other substances and promotes tumor proliferation. PKC is a key component in biological pathways controlling cell growth and differentiation. When phorbol esters bind to PKC, cell proliferation pathways are activated. This effect greatly promotes tumors when the cells are exposed to even a sub-carcinogenic amount of a substance.
PKC is also involved in activation of inflammation pathways such as the NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a family of transcription factor protein complexes that controls transcription (genetics), transcription of DNA, cytokine production and cell survival. NF-κB is found i ...
pathway. Thus, exposure to phorbol products can induce an inflammatory response in tissues. Symptoms can include edema and pain, especially to the skin and mucous membranes.
While phorbol itself does not have irritant activity, nearly all phorbol esters are highly irritant, with a wide range of half-maximal inhibitory concentration ( IC50) values. The median lethal dose ( LD50) of phorbol esters for male mice was found to be about 27 mg/kg, with the mice showing hemorrhage and congestion of pulmonary blood vessels, as well as lesions throughout the body.
Total synthesis
Atotal synthesis
Total synthesis, a specialized area within organic chemistry, focuses on constructing complex organic compounds, especially those found in nature, using laboratory methods. It often involves synthesizing natural products from basic, commercially ...
of enantiopure
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities which are mirror images of each other and non-superpos ...
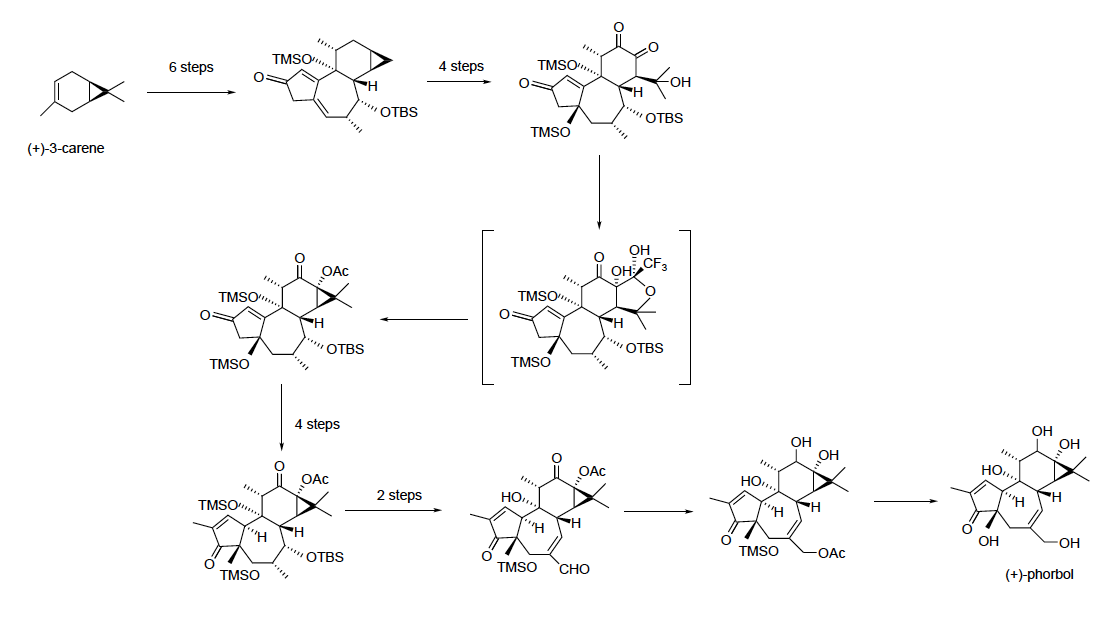
phorbol was developed in 2015. While this synthesis will not replace natural isolation products, it will enable researchers to create phorbol analogs for use in research, especially creating phorbol derivatives that can be evaluated for anti-cancer activity. Previously, the difficulty with synthesizing phorbol had been creating C–C bonds, especially in the six-membered ring at the top of the molecule. This synthesis starts from (+)- 3-carene, and uses a series of 19 steps to eventually create (+)-phorbol.
:
Uses in biomedical research
Because of their mechanism of action, phorbol esters can be used to study tumor proliferation and pain response. TPA is most commonly used in the laboratory to induce a cellular response. For example, TPA can be used to measure response to pain and test compounds that may mitigate the inflammatory response. TPA and other phorbol esters can also be used to induce tumor formation and to study mechanism of action. TPA, together with ionomycin, can also be used to stimulate T-cell activation, proliferation, and cytokine production, and is used in protocols for intracellular staining of these cytokines.Possible and purported medicinal uses
The phorbol ester tigilanol tiglate reportedly has ''in vitro'' anti-cancer, antiviral, and antibacterial activities. The phorbol derivatives in croton oil are used in folk medicine, with purported purgative, counter-irritant, oranthelmintic
Anthelmintics or antihelminthics are a group of antiparasitic drugs that expel parasitic worms (helminths) and other internal parasites from the body by either stunning or killing them without causing significant damage to the host. They may also ...
activities.
References
Further reading
* *External links
* {{Commons category, Phorbols Diterpenes Alcohols Benzoazulenes Ketones Total synthesis Cyclopropanes Cyclopentenes Protein kinase C activators Phorbol esters