phenolic insulation on:
[Wikipedia]
[Google]
[Amazon]
Phenol formaldehyde resins (PF), also called phenolic resins or phenoplasts, are synthetic polymers obtained by the reaction of
 Novolaks (or novolacs) are phenol-formaldehyde resins with a formaldehyde to phenol molar ratio of less than one. In place of phenol itself, they are often produced from cresols (methylphenols). The polymerization is brought to completion using acid-catalysis such as
Novolaks (or novolacs) are phenol-formaldehyde resins with a formaldehyde to phenol molar ratio of less than one. In place of phenol itself, they are often produced from cresols (methylphenols). The polymerization is brought to completion using acid-catalysis such as
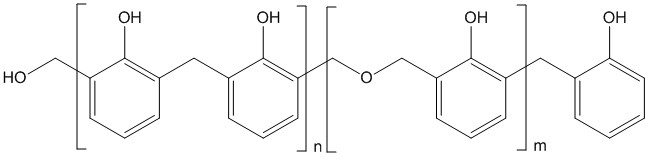 Base-catalysed phenol-formaldehyde resins are made with a formaldehyde to phenol ratio of greater than one (usually around 1.5). These resins are called resoles. Phenol, formaldehyde, water and catalyst are mixed in the desired amount, depending on the resin to be formed, and are then heated. The first part of the reaction, at around 70 °C, forms a thick reddish-brown tacky material, which is rich in hydroxymethyl and benzylic ether groups.
The rate of the base-catalysed reaction initially increases with pH, and reaches a maximum at about pH = 10. The reactive species is the phenoxide anion (C6H5O−) formed by deprotonation of phenol. The negative charge is delocalised over the aromatic ring, activating sites 2, 4 and 6, which then react with the formaldehyde.
Being thermosets, hydroxymethyl phenols will crosslink on heating to around 120 °C to form methylene and methyl ether bridges through the elimination of water molecules. At this point the resin is a 3-dimensional network, which is typical of polymerised phenolic resins. The high crosslinking gives this type of phenolic resin its hardness, good thermal stability, and chemical imperviousness. Resoles are referred to as "one step" resins as they cure without a cross linker unlike novolacs, a "two step" resin.
Resoles are major polymeric resin materials widely used for gluing and bonding building materials. Exterior plywood, oriented strand boards (OSB), engineered high-pressure laminate are typical applications.
Base-catalysed phenol-formaldehyde resins are made with a formaldehyde to phenol ratio of greater than one (usually around 1.5). These resins are called resoles. Phenol, formaldehyde, water and catalyst are mixed in the desired amount, depending on the resin to be formed, and are then heated. The first part of the reaction, at around 70 °C, forms a thick reddish-brown tacky material, which is rich in hydroxymethyl and benzylic ether groups.
The rate of the base-catalysed reaction initially increases with pH, and reaches a maximum at about pH = 10. The reactive species is the phenoxide anion (C6H5O−) formed by deprotonation of phenol. The negative charge is delocalised over the aromatic ring, activating sites 2, 4 and 6, which then react with the formaldehyde.
Being thermosets, hydroxymethyl phenols will crosslink on heating to around 120 °C to form methylene and methyl ether bridges through the elimination of water molecules. At this point the resin is a 3-dimensional network, which is typical of polymerised phenolic resins. The high crosslinking gives this type of phenolic resin its hardness, good thermal stability, and chemical imperviousness. Resoles are referred to as "one step" resins as they cure without a cross linker unlike novolacs, a "two step" resin.
Resoles are major polymeric resin materials widely used for gluing and bonding building materials. Exterior plywood, oriented strand boards (OSB), engineered high-pressure laminate are typical applications.
 *
*
phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
or substituted phenol with formaldehyde. Used as the basis for Bakelite
Bakelite ( ), formally , is a thermosetting polymer, thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. The first plastic made from synthetic components, it was developed by Belgian chemist ...
, PFs were the first commercial synthetic resins. They have been widely used for the production of molded products including billiard balls, laboratory countertops, and as coatings and adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.
The use of adhesives offers certain advantage ...
s. They were at one time the primary material used for the production of circuit board
A printed circuit board (PCB), also called printed wiring board (PWB), is a laminated sandwich structure of conductive and insulating layers, each with a pattern of traces, planes and other features (similar to wires on a flat surface) ...
s but have been largely replaced with epoxy resins and fiberglass
Fiberglass (American English) or fibreglass (English in the Commonwealth of Nations, Commonwealth English) is a common type of fibre-reinforced plastic, fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened i ...
cloth, as with fire-resistant FR-4 circuit board materials.
There are two main production methods. One reacts phenol and formaldehyde directly to produce a thermosetting network polymer, while the other restricts the formaldehyde to produce a prepolymer known as novolac which can be moulded and then cured with the addition of more formaldehyde and heat.A. Gardziella, L.A. Pilato, A. Knop, Phenolic Resins: Chemistry, Applications, Standardization, Safety and Ecology, 2nd edition, Springer, 2000 There are many variations in both production and input materials that are used to produce a wide variety of resins for special purposes.
Formation and structure
Phenol-formaldehyde resins, as a group, are formed by a step-growth polymerization reaction that can be eitheracid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
- or base-catalysed. Since formaldehyde exists predominantly in solution as a dynamic equilibrium of methylene glycol oligomers, the concentration of the ''reactive'' form of formaldehyde depends on temperature and pH.
Phenol reacts with formaldehyde at the ortho and para sites (sites 2, 4 and 6) allowing up to 3 units of formaldehyde to attach to the ring. The initial reaction in all cases involves the formation of a hydroxymethyl phenol:
: HOC6H5 + CH2O → HOC6H4CH2OH
The hydroxymethyl group is capable of reacting with either another free ortho or para site, or with another hydroxymethyl group. The first reaction gives a methylene bridge, and the second forms an ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
bridge:
: HOC6H4CH2OH + HOC6H5 → (HOC6H4)2CH2 + H2O
: 2 HOC6H4CH2OH → (HOC6H4CH2)2O + H2O
The diphenol (HOC6H4)2CH2 (sometimes called a "dimer") is called bisphenol F, which is an important monomer in the production of epoxy resins. Bisphenol-F can further link generating tri- and tetra-and higher phenol oligomers.
Novolaks
 Novolaks (or novolacs) are phenol-formaldehyde resins with a formaldehyde to phenol molar ratio of less than one. In place of phenol itself, they are often produced from cresols (methylphenols). The polymerization is brought to completion using acid-catalysis such as
Novolaks (or novolacs) are phenol-formaldehyde resins with a formaldehyde to phenol molar ratio of less than one. In place of phenol itself, they are often produced from cresols (methylphenols). The polymerization is brought to completion using acid-catalysis such as sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
, oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name i ...
, hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
and rarely, sulfonic acids. The phenolic units are mainly linked by methylene and/or ether groups. The molecular weights are in the low thousands, corresponding to about 10–20 phenol units. Obtained polymer is thermoplastic and require a curing agent or hardener to form a thermoset
In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening (" curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and ...
.
Hexamethylenetetramine is a hardener added to crosslink novolac. At a temperature greater than 90 °C, it forms methylene and dimethylene amino bridges. Resoles can also be used as a curing agent (hardener) for novolac resins. In either case, the curing agent is a source of formaldehyde which provides bridges between novolac chains, eventually completely crosslinking the system.
Novolacs have multiple uses as tire tackifier, high temperature resin, binder for carbon bonded refractories, carbon brakes, photoresist
A photoresist (also known simply as a resist) is a light-sensitive material used in several processes, such as photolithography and photoengraving, to form a patterned coating on a surface. This process is crucial in the electronics industry.
T ...
s and as a curing agent for epoxy resins.
Resoles
 Base-catalysed phenol-formaldehyde resins are made with a formaldehyde to phenol ratio of greater than one (usually around 1.5). These resins are called resoles. Phenol, formaldehyde, water and catalyst are mixed in the desired amount, depending on the resin to be formed, and are then heated. The first part of the reaction, at around 70 °C, forms a thick reddish-brown tacky material, which is rich in hydroxymethyl and benzylic ether groups.
The rate of the base-catalysed reaction initially increases with pH, and reaches a maximum at about pH = 10. The reactive species is the phenoxide anion (C6H5O−) formed by deprotonation of phenol. The negative charge is delocalised over the aromatic ring, activating sites 2, 4 and 6, which then react with the formaldehyde.
Being thermosets, hydroxymethyl phenols will crosslink on heating to around 120 °C to form methylene and methyl ether bridges through the elimination of water molecules. At this point the resin is a 3-dimensional network, which is typical of polymerised phenolic resins. The high crosslinking gives this type of phenolic resin its hardness, good thermal stability, and chemical imperviousness. Resoles are referred to as "one step" resins as they cure without a cross linker unlike novolacs, a "two step" resin.
Resoles are major polymeric resin materials widely used for gluing and bonding building materials. Exterior plywood, oriented strand boards (OSB), engineered high-pressure laminate are typical applications.
Base-catalysed phenol-formaldehyde resins are made with a formaldehyde to phenol ratio of greater than one (usually around 1.5). These resins are called resoles. Phenol, formaldehyde, water and catalyst are mixed in the desired amount, depending on the resin to be formed, and are then heated. The first part of the reaction, at around 70 °C, forms a thick reddish-brown tacky material, which is rich in hydroxymethyl and benzylic ether groups.
The rate of the base-catalysed reaction initially increases with pH, and reaches a maximum at about pH = 10. The reactive species is the phenoxide anion (C6H5O−) formed by deprotonation of phenol. The negative charge is delocalised over the aromatic ring, activating sites 2, 4 and 6, which then react with the formaldehyde.
Being thermosets, hydroxymethyl phenols will crosslink on heating to around 120 °C to form methylene and methyl ether bridges through the elimination of water molecules. At this point the resin is a 3-dimensional network, which is typical of polymerised phenolic resins. The high crosslinking gives this type of phenolic resin its hardness, good thermal stability, and chemical imperviousness. Resoles are referred to as "one step" resins as they cure without a cross linker unlike novolacs, a "two step" resin.
Resoles are major polymeric resin materials widely used for gluing and bonding building materials. Exterior plywood, oriented strand boards (OSB), engineered high-pressure laminate are typical applications.
Crosslinking and the formaldehyde/phenol ratio
When the molar ratio of formaldehyde:phenol reaches one, in theory every phenol is linked together via methylene bridges, generating one single molecule, and the system is entirely crosslinked. This is why novolacs (F:P <1) do not harden without the addition of a crosslinking agents, and why resoles with the formula F:P >1 will.Applications
Phenolic resins are found in myriad industrial products. Phenoliclaminate
Simulated flight (using image stack created by μCT scanning) through the length of a knitting needle that consists of laminated wooden layers: the layers can be differentiated by the change of direction of the wood's vessels
Shattered windshi ...
s are made by impregnating one or more layers of a base material such as paper, fiberglass
Fiberglass (American English) or fibreglass (English in the Commonwealth of Nations, Commonwealth English) is a common type of fibre-reinforced plastic, fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened i ...
, or cotton with phenolic resin and laminating the resin-saturated base material under heat and pressure. The resin fully polymerize
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form ...
s (cures) during this process forming the thermoset polymer matrix. The base material choice depends on the intended application of the finished product. Paper phenolics are used in manufacturing electrical components such as punch-through boards, in household laminates, and in paper composite panels. Glass phenolics are particularly well suited for use in the high speed bearing market. Phenolic micro-balloons are used for density control. The binding agent in normal (organic) brake pads, brake shoes, and clutch discs are phenolic resin. Synthetic resin bonded paper, made from phenolic resin and paper, is used to make countertops. Another use of phenolic resins is the making of duroplast, famously used in Trabant automobiles.
Phenolic resins are also used for making exterior plywood commonly known as ''weather and boil proof'' (WBP) plywood because phenolic resins have no melting point but only a decomposing point in the temperature zone of and above.
Phenolic resin is used as a binder in loudspeaker
A loudspeaker (commonly referred to as a speaker or, more fully, a speaker system) is a combination of one or more speaker drivers, an enclosure, and electrical connections (possibly including a crossover network). The speaker driver is an ...
driver suspension components which are made of cloth
Textile is an umbrella term that includes various fiber-based materials, including fibers, yarns, filaments, threads, and different types of fabric. At first, the word "textiles" only referred to woven fabrics. However, weaving is n ...
.
Higher end billiard balls are made from phenolic resins, as opposed to the polyesters used in less expensive sets.
Sometimes people select fibre reinforced phenolic resin parts because their coefficient of thermal expansion closely matches that of the aluminium used for other parts of a system, as in early computer systems
and Duramold.
The Dutch painting forger Han van Meegeren
Henricus Antonius "Han" van Meegeren (; 10 October 1889 – 30 December 1947) was a Dutch painter and portraitist, considered one of the most ingenious Art forgery, art forgers of the 20th century. Van Meegeren became a national hero after World ...
mixed phenol formaldehyde with his oil paints before baking the finished canvas, in order to fake the drying out of the paint over the centuries.
Atmospheric re-entry spacecraft use phenol formaldehyde resin as a key component in ablative heat shields (e.g. AVCOAT
AVCOAT 5026-39 is a NASA code for two versions of a specific Atmospheric reentry#Ablative, ablative heat shield material originally created by Avco for the Apollo program.
It is composed of silica fibers in an epoxy novolac resin. The original AVC ...
on the Apollo modules). As the heat shield skin temperature can reach 1000-2000 °C, the resin pyrolizes due to aerodynamic heating. This reaction absorbs significant thermal energy, insulating the deeper layers of the heat shield. The outgassing of pyrolisis reaction products and the removal of charred material by friction (ablation) also contribute to vehicle insulation, by mechanically carrying away the heat absorbed in those materials.
Trade names
 *
* Bakelite
Bakelite ( ), formally , is a thermosetting polymer, thermosetting phenol formaldehyde resin, formed from a condensation reaction of phenol with formaldehyde. The first plastic made from synthetic components, it was developed by Belgian chemist ...
was originally made from phenolic resin and wood flour.
* Ebonol is a paper-filled phenolic resin designed as a replacement for ebony wood in stringed and woodwind
Woodwind instruments are a family of musical instruments within the greater category of wind instruments.
Common examples include flute, clarinet, oboe, bassoon, and saxophone. There are two main types of woodwind instruments: flutes and Ree ...
instruments.
* Novotext is cotton fibre-reinforced phenolic, using randomly oriented fibres.
** Tufnol is a laminated plastic available as sheet and rods, which is made from layers of paper or cloth which have been soaked with phenolic resin and pressed under heat. Its high resistance to oils and solvents have made it suitable for many engineering applications.
* Oasis
In ecology, an oasis (; : oases ) is a fertile area of a desert or semi-desert environmentPaxolin is a resin bonded paper product long used as a base material for printed circuit boards, although it is being replaced by fiberglass composites in many applications.
* Richlite is a paper-filled phenolic resin with many uses, from tabletops and cutting-boards to guitar fingerboards.
Biodegradation
Phenol-formaldehyde is degraded by the white rot fungus ''Phanerochaete chrysosporium
''Phanerochaete'' is a genus of crust fungi in the family Phanerochaetaceae.
Taxonomy
The genus was circumscription (taxonomy), circumscribed by Finnish mycologist Petter Karsten in 1889. Marinus Anton Donk redefined the limits of the genus in t ...
''.
See also
* Urea-formaldehyde * Para tertiary butylphenol formaldehyde resinReferences
{{Organic reactions Synthetic resins Semiconductor device fabrication Thermosetting plastics