Oceanic Carbon Cycle on:
[Wikipedia]
[Google]
[Amazon]
 The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange
The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange
 Carbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as –
Carbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as –
 The oceans store the largest pool of reactive carbon on the planet as DIC, which is introduced as a result of the dissolution of atmospheric carbon dioxide into seawater – the solubility pump. Aqueous CO2, carbonic acid, bicarbonate ion, and carbonate ion concentrations comprise dissolved inorganic carbon (DIC). DIC circulates throughout the whole ocean by Thermohaline circulation, which facilitates the tremendous DIC storage capacity of the ocean. The below chemical equations show the reactions that CO2 undergoes after it enters the ocean and transforms into its aqueous form.
The oceans store the largest pool of reactive carbon on the planet as DIC, which is introduced as a result of the dissolution of atmospheric carbon dioxide into seawater – the solubility pump. Aqueous CO2, carbonic acid, bicarbonate ion, and carbonate ion concentrations comprise dissolved inorganic carbon (DIC). DIC circulates throughout the whole ocean by Thermohaline circulation, which facilitates the tremendous DIC storage capacity of the ocean. The below chemical equations show the reactions that CO2 undergoes after it enters the ocean and transforms into its aqueous form.
 Carbonic acid rapidly dissociates into free
Carbonic acid rapidly dissociates into free
 Before the
Before the
 The key outputs of the marine carbon system are particulate organic matter (POC) and calcium carbonate (PIC) preservation as well as reverse weathering. While there are regions with local loss of CO2 to the atmosphere and hydrothermal processes, a net loss in the cycle does not occur.
The key outputs of the marine carbon system are particulate organic matter (POC) and calcium carbonate (PIC) preservation as well as reverse weathering. While there are regions with local loss of CO2 to the atmosphere and hydrothermal processes, a net loss in the cycle does not occur.
 Text and images are available under
Text and images are available under
Creative Commons Attribution 4.0 International License
This may be beneficial in terms of
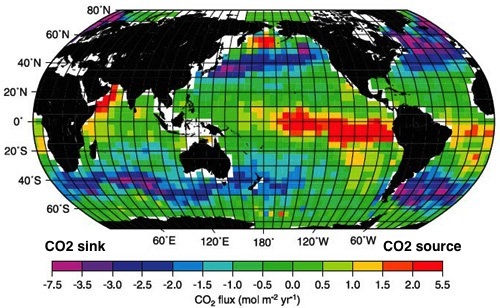
Current global map of the partial pressure of carbon dioxide at the ocean surfaceCurrent global map of the sea-air carbon dioxide flux density
{{biogeochemical cycle Carbon cycle Chemical oceanography
 The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange
The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle
The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major compon ...
is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemist ...
carbon (carbon not associated with a living thing, such as carbon dioxide) and organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
carbon (carbon that is, or has been, incorporated into a living thing). Part of the marine carbon cycle transforms carbon between non-living and living matter.
Three main processes (or pumps) that make up the marine carbon cycle bring atmospheric carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
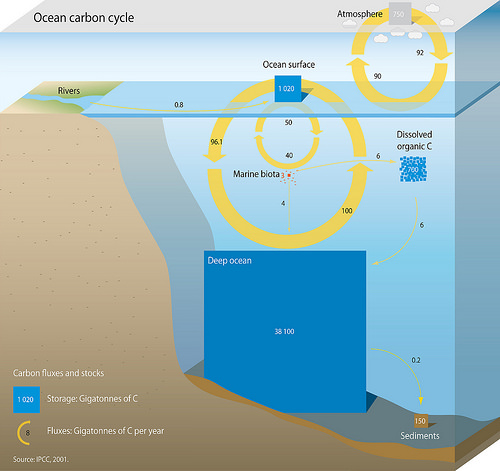
(CO2) into the ocean interior and distribute it through the oceans. These three pumps are: (1) the solubility pump, (2) the carbonate pump, and (3) the biological pump. The total active pool of carbon at the Earth's surface for durations of less than 10,000 years is roughly 40,000 gigatons C (Gt C, a gigaton is one billion tons, or the weight of approximately 6 million blue whale
The blue whale (''Balaenoptera musculus'') is a marine mammal and a baleen whale. Reaching a maximum confirmed length of and weighing up to , it is the largest animal known to have ever existed. The blue whale's long and slender body can ...
s), and about 95% (~38,000 Gt C) is stored in the ocean, mostly as dissolved inorganic carbon. The speciation of dissolved inorganic carbon in the marine carbon cycle is a primary controller of acid-base chemistry in the oceans.
Earth's plants and algae ( primary producers) are responsible for the largest annual carbon fluxes. Although the amount of carbon stored in marine biota (~3 Gt C) is very small compared with terrestrial vegetation (~610 GtC), the amount of carbon exchanged (the flux) by these groups is nearly equal – about 50 GtC each. Marine organisms link the carbon and oxygen cycles through processes such as photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in ...
. The marine carbon cycle is also biologically tied to the nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
cycles by a near-constant stoichiometric ratio C:N:P of 106:16:1, also known as the Redfield Ketchum Richards (RKR) ratio, which states that organisms tend to take up nitrogen and phosphorus incorporating new organic carbon. Likewise, organic matter decomposed by bacteria releases phosphorus and nitrogen.
Based on the publications of NASA
The National Aeronautics and Space Administration (NASA ) is an independent agency of the US federal government responsible for the civil space program, aeronautics research, and space research.
NASA was established in 1958, succeedin ...
, World Meteorological Association, IPCC
The Intergovernmental Panel on Climate Change (IPCC) is an intergovernmental body of the United Nations. Its job is to advance scientific knowledge about climate change caused by human activities. The World Meteorological Organization (WMO) ...
, and International Council for the Exploration of the Sea
The International Council for the Exploration of the Sea (ICES; french: Conseil International de l'Exploration de la Mer, ''CIEM'') is a regional fishery advisory body and the world's oldest intergovernmental science organization. ICES is headqua ...
, as well as scientists from NOAA
The National Oceanic and Atmospheric Administration (abbreviated as NOAA ) is an United States scientific and regulatory agency within the United States Department of Commerce that forecasts weather, monitors oceanic and atmospheric conditio ...
, Woods Hole Oceanographic Institution
The Woods Hole Oceanographic Institution (WHOI, acronym pronounced ) is a private, nonprofit research and higher education facility dedicated to the study of marine science and engineering.
Established in 1930 in Woods Hole, Massachusetts, i ...
, Scripps Institution of Oceanography
The Scripps Institution of Oceanography (sometimes referred to as SIO, Scripps Oceanography, or Scripps) in San Diego, California, US founded in 1903, is one of the oldest and largest centers for ocean and Earth science research, public servi ...
, CSIRO
The Commonwealth Scientific and Industrial Research Organisation (CSIRO) is an Australian Government agency responsible for scientific research.
CSIRO works with leading organisations around the world. From its headquarters in Canberra, CSIRO ...
, and Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research an ...
, the human impacts on the marine carbon cycle are significant. Before the Industrial Revolution, the ocean was a net source of CO2 to the atmosphere whereas now the majority of the carbon that enters the ocean comes from atmospheric carbon dioxide (CO2). The burning of fossil fuels and production of cement have changed the balance of carbon dioxide between the atmosphere and oceans, causing acidification of the oceans. Climate change, a result of excess CO2 in the atmosphere, has increased the temperature of the ocean and atmosphere (global warming
In common usage, climate change describes global warming—the ongoing increase in global average temperature—and its effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes to ...
). The slowed rate of global warming occurring from 2000–2010 may be attributed to an observed increase in upper ocean heat content
In oceanography and climatology, ocean heat content (OHC) is a term for the energy absorbed by the ocean, where it is stored for indefinite time periods as internal energy or enthalpy. The rise in OHC accounts for over 90% of Earth’s excess t ...
.
Marine carbon
 Carbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as –
Carbon compounds can be distinguished as either organic or inorganic, and dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
s, lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids in ...
s, carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may o ...
s, and nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main ...
s. Inorganic carbon is found primarily in simple compounds such as carbon dioxide, carbonic acid, bicarbonate, and carbonate (CO2, H2CO3, HCO3−, CO32− respectively).
Marine carbon is further separated into particulate and dissolved phases. These pools are operationally defined by physical separation – dissolved carbon passes through a 0.2 μm filter, and particulate carbon does not.
Inorganic carbon
There are two main types of inorganic carbon that are found in the oceans. Dissolved inorganic carbon (DIC) is made up of bicarbonate (HCO3−), carbonate (CO32−) and carbon dioxide (including both dissolved CO2 and carbonic acid H2CO3). DIC can be converted toparticulate inorganic carbon
Particulate inorganic carbon (PIC) can be contrasted with dissolved inorganic carbon (DIC), the other form of inorganic carbon found in the ocean. These distinctions are important in chemical oceanography. Particulate inorganic carbon is somet ...
(PIC) through precipitation of CaCO3 (biologically or abiotically). DIC can also be converted to particulate organic carbon (POC) through photosynthesis and chemoautotrophy
A Chemotroph is an organism that obtains energy by the oxidation of electron donors in their environments. These molecules can be organic (chemoorganotrophs) or inorganic (chemolithotrophs). The chemotroph designation is in contrast to phototro ...
(i.e. primary production). DIC increases with depth as organic carbon particles sink and are respired. Free oxygen decreases as DIC increases because oxygen is consumed during aerobic respiration.
Particulate inorganic carbon (PIC) is the other form of inorganic carbon found in the ocean. Most PIC is the CaCO3 that makes up shells of various marine organisms, but can also form in whiting events. Marine fish also excrete calcium carbonate during osmoregulation
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration o ...
.
Some of the inorganic carbon species in the ocean, such as bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemi ...
and carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
, are major contributors to alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength ...
, a natural ocean buffer that prevents drastic changes in acidity (or pH). The marine carbon cycle also affects the reaction and dissolution rates of some chemical compounds, regulates the amount of carbon dioxide in the atmosphere and Earth's temperature.
Organic carbon
Like inorganic carbon, there are two main forms of organic carbon found in the ocean (dissolved and particulate). Dissolved organic carbon (DOC) is defined operationally as any organic molecule that can pass through a 0.2 µm filter. DOC can be converted into particulate organic carbon through heterotrophy and it can also be converted back to dissolved inorganic carbon (DIC) through respiration. Those organic carbon molecules being captured on a filter are defined as particulate organic carbon (POC). POC is composed of organisms (dead or alive), their fecal matter, anddetritus
In biology, detritus () is dead particulate organic material, as distinguished from dissolved organic material. Detritus typically includes the bodies or fragments of bodies of dead organisms, and fecal material. Detritus typically hosts comm ...
. POC can be converted to DOC through disaggregation of molecules and by exudation by phytoplankton
Phytoplankton () are the autotrophic (self-feeding) components of the plankton community and a key part of ocean and freshwater ecosystems. The name comes from the Greek words (), meaning 'plant', and (), meaning 'wanderer' or 'drifter'.
...
, for example. POC is generally converted to DIC through heterotrophy and respiration.
Marine carbon pumps
Solubility pump
''Full article: Solubility pump'' The oceans store the largest pool of reactive carbon on the planet as DIC, which is introduced as a result of the dissolution of atmospheric carbon dioxide into seawater – the solubility pump. Aqueous CO2, carbonic acid, bicarbonate ion, and carbonate ion concentrations comprise dissolved inorganic carbon (DIC). DIC circulates throughout the whole ocean by Thermohaline circulation, which facilitates the tremendous DIC storage capacity of the ocean. The below chemical equations show the reactions that CO2 undergoes after it enters the ocean and transforms into its aqueous form.
The oceans store the largest pool of reactive carbon on the planet as DIC, which is introduced as a result of the dissolution of atmospheric carbon dioxide into seawater – the solubility pump. Aqueous CO2, carbonic acid, bicarbonate ion, and carbonate ion concentrations comprise dissolved inorganic carbon (DIC). DIC circulates throughout the whole ocean by Thermohaline circulation, which facilitates the tremendous DIC storage capacity of the ocean. The below chemical equations show the reactions that CO2 undergoes after it enters the ocean and transforms into its aqueous form.
 Carbonic acid rapidly dissociates into free
Carbonic acid rapidly dissociates into free hydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particle ...
(technically, hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid ...
) and bicarbonate.The free hydrogen ion meets carbonate, already present in the water from the dissolution of CaCO3, and reacts to form more bicarbonate ion.The dissolved species in the equations above, mostly bicarbonate, make up the carbonate alkalinity system, the dominant contributor to seawater alkalinity.
Carbonate pump
The carbonate pump, sometimes called the carbonate counter pump, starts with marine organisms at the ocean's surface producing particulate inorganic carbon (PIC) in the form of calcium carbonate (calcite
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on scratc ...
or aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including pre ...
, CaCO3). This CaCO3 is what forms hard body parts like shells. The formation of these shells increases atmospheric CO2 due to the production of CaCO3 in the following reaction with simplified stoichiometry:Coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the king ...
s, a nearly ubiquitous group of phytoplankton that produce shells of calcium carbonate, are the dominant contributors to the carbonate pump. Due to their abundance, coccolithophores have significant implications on carbonate chemistry, in the surface waters they inhabit and in the ocean below: they provide a large mechanism for the downward transport of CaCO3. The air-sea CO2 flux induced by a marine biological community
A community is a social unit (a group of living things) with commonality such as place, norms, religion, values, customs, or identity. Communities may share a sense of place situated in a given geographical area (e.g. a country, village, t ...
can be determined by the rain ratio - the proportion of carbon from calcium carbonate compared to that from organic carbon in particulate matter sinking to the ocean floor, (PIC/POC). The carbonate pump acts as a negative feedback on CO2 taken into the ocean by the solubility pump. It occurs with lesser magnitude than the solubility pump.
Biological pump
''Full article:Biological pump
The biological pump (or ocean carbon biological pump or marine biological carbon pump) is the ocean's biologically driven sequestration of carbon from the atmosphere and land runoff to the ocean interior and seafloor sediments.Sigman DM & GH ...
''
Particulate organic carbon, created through biological production, can be exported from the upper ocean in a flux commonly termed the biological pump, or respired (equation 6) back into inorganic carbon. In the former, dissolved inorganic carbon is biologically converted into organic matter by photosynthesis (equation 5) and other forms of autotroph
An autotroph or primary producer is an organism that produces complex organic compounds (such as carbohydrates, fats, and proteins) using carbon from simple substances such as carbon dioxide,Morris, J. et al. (2019). "Biology: How Life Wo ...
y that then sinks and is, in part or whole, digested by heterotrophs. Particulate organic carbon can be classified, based on how easily organisms can break them down for food, as labile, semilabile, or refractory. Photosynthesis by phytoplankton is the primary source for labile and semilabile molecules, and is the indirect source for most refractory molecules. Labile molecules are present at low concentrations outside of cells (in the picomolar
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular of a solute in a solution, in terms of amount of substance per unit volume of sol ...
range) and have half-lives of only minutes when free in the ocean. They are consumed by microbes within hours or days of production and reside in the surface oceans, where they contribute a majority of the labile carbon flux. Semilabile molecules, much more difficult to consume, are able to reach depths of hundreds of meters below the surface before being metabolized. Refractory DOM largely comprises highly conjugated molecules like Polycyclic aromatic hydrocarbon
A polycyclic aromatic hydrocarbon (PAH) is a class of organic compounds that is composed of multiple aromatic rings. The simplest representative is naphthalene, having two aromatic rings and the three-ring compounds anthracene and phenanthrene. ...
s or lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity a ...
. Refractory DOM can reach depths greater than 1000 m and circulates through the oceans over thousands of years. Over the course of a year, approximately 20 gigatons of photosynthetically-fixed labile and semilabile carbon is taken up by heterotroph
A heterotroph (; ) is an organism that cannot produce its own food, instead taking nutrition from other sources of organic carbon, mainly plant or animal matter. In the food chain, heterotrophs are primary, secondary and tertiary consumers, but ...
s, whereas fewer than 0.2 gigatons of refractory carbon is consumed. Marine dissolved organic matter
Dissolved organic carbon (DOC) is the fraction of organic carbon operationally defined as that which can pass through a filter with a pore size typically between 0.22 and 0.7 micrometers. The fraction remaining on the filter is called parti ...
(DOM) can store as much carbon as the current atmospheric CO2 supply, but industrial processes are altering the balance of this cycle.
Inputs
Inputs to the marine carbon cycle are numerous, but the primary contributions, on a net basis, come from the atmosphere and rivers.Hydrothermal vent
A hydrothermal vent is a fissure on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hotspo ...
s generally supply carbon equal to the amount they consume.
Atmosphere

 Before the
Before the Industrial Revolution
The Industrial Revolution was the transition to new manufacturing processes in Great Britain, continental Europe, and the United States, that occurred during the period from around 1760 to about 1820–1840. This transition included going f ...
, the ocean was a source of CO2 to the atmosphere
An atmosphere () is a layer of gas or layers of gases that envelop a planet, and is held in place by the gravity of the planetary body. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A ...
balancing the impact of rock weathering and terrestrial particulate organic carbon; now it has become a sink for the excess atmospheric CO2. Carbon dioxide is absorbed from the atmosphere at the ocean's surface at an exchange rate which varies locally but on average, the oceans have a net absorption of CO2 2.2 Pg C per year. Because the solubility of carbon dioxide increases when temperature decreases, cold areas can contain more CO2 and still be in equilibrium with the atmosphere; In contrast, rising sea surface temperatures decrease the capacity of the oceans to take in carbon dioxide. The North Atlantic
The Atlantic Ocean is the second-largest of the world's five oceans, with an area of about . It covers approximately 20% of Earth's surface and about 29% of its water surface area. It is known to separate the " Old World" of Africa, Europe ...
and Nordic oceans have the highest carbon uptake per unit area in the world, and in the North Atlantic deep convection transports approximately 197 Tg per year of non-refractory carbon to depth.
Carbon dioxide exchange rates between ocean and atmosphere
Ocean-atmospheric exchanges rates of CO2 depend on the concentration of carbon dioxide already present in both the atmosphere and the ocean, temperature, salinity, and wind speed. This exchange rate can be approximated by Henry's law and can be calculated as S = kP, where thesolubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
(S) of the carbon dioxide gas is proportional to the amount of gas in the atmosphere, or its partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
.
Revelle factor
Since the oceanic intake of carbon dioxide is limited, CO2 influx can also be described by the Revelle factor. The Revelle Factor is a ratio of the change of carbon dioxide to the change in dissolved inorganic carbon, which serves as an indicator of carbon dioxide dissolution in the mixed layer considering the solubility pump. The Revelle Factor is an expression to characterize the thermodynamic efficiency of the DIC pool to absorb CO2 into bicarbonate. The lower the Revelle factor, the higher the capacity for ocean water to take in carbon dioxide. While Revelle calculated a factor of around 10 in his day, in a 2004 study data showed a Revelle factor ranging from approximately 9 in low-latitude tropical regions to 15 in the southern ocean near Antarctica.Rivers
River
A river is a natural flowing watercourse, usually freshwater, flowing towards an ocean, sea, lake or another river. In some cases, a river flows into the ground and becomes dry at the end of its course without reaching another body of ...
s can also transport organic carbon to the ocean through weathering
Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs '' in situ'' (on site, with little or no movement ...
or erosion of aluminosilicate (equation 7) and carbonate rocks (equation 8) on land,
or by the decomposition of life (equation 5, e.g. plant and soil material). Rivers contribute roughly equal amounts (~0.4 GtC/yr) of DIC and DOC to the oceans. It is estimated that approximately 0.8 GtC (DIC + DOC) is transported annually from the rivers to the ocean. The rivers that flow into Chesapeake Bay
The Chesapeake Bay ( ) is the largest estuary in the United States. The Bay is located in the Mid-Atlantic region and is primarily separated from the Atlantic Ocean by the Delmarva Peninsula (including the parts: the Eastern Shore of Maryland / ...
( Susquehanna, Potomac, and James rivers) input approximately 0.004 Gt (6.5 x 1010 moles) DIC per year. The total carbon transport of rivers represents approximately 0.02% of the total carbon in the atmosphere. Though it seems small, over long time scales (1000 to 10,000 years) the carbon that enters rivers (and therefore does not enter the atmosphere) serves as a stabilizing feedback for greenhouse
A greenhouse (also called a glasshouse, or, if with sufficient heating, a hothouse) is a structure with walls and roof made chiefly of transparent material, such as glass, in which plants requiring regulated climatic conditions are grown.These ...
warming.
Outputs
 The key outputs of the marine carbon system are particulate organic matter (POC) and calcium carbonate (PIC) preservation as well as reverse weathering. While there are regions with local loss of CO2 to the atmosphere and hydrothermal processes, a net loss in the cycle does not occur.
The key outputs of the marine carbon system are particulate organic matter (POC) and calcium carbonate (PIC) preservation as well as reverse weathering. While there are regions with local loss of CO2 to the atmosphere and hydrothermal processes, a net loss in the cycle does not occur.
Organic matter preservation
Sedimentation is a long-term sink for carbon in the ocean, as well as the largest loss of carbon from the oceanic system. Deepmarine sediment
Marine sediment, or ocean sediment, or seafloor sediment, are deposits of insoluble particles that have accumulated on the seafloor. These particles have their origins in soil and rocks and have been transported from the land to the sea, mai ...
s and geologic formation
A geological formation, or simply formation, is a body of rock having a consistent set of physical characteristics (lithology) that distinguishes it from adjacent bodies of rock, and which occupies a particular position in the layers of rock expo ...
s are important since they provide a thorough record of life on Earth and an important source of fossil fuel. Oceanic carbon can exit the system in the form of detritus that sinks and is buried in the seafloor without being fully decomposed or dissolved. Ocean floor surface sediment
Sediment is a naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of wind, water, or ice or by the force of gravity acting on the particles. For example, sand ...
s account for 1.75x1015 kg of carbon in the global carbon cycle At most, 4% of the particulate organic carbon from the euphotic
The photic zone, euphotic zone, epipelagic zone, or sunlight zone is the uppermost layer of a body of water that receives sunlight, allowing phytoplankton to perform photosynthesis. It undergoes a series of physical, chemical, and biological pro ...
zone in the Pacific Ocean, where light-powered primary production
In ecology, primary production is the synthesis of organic compounds from atmospheric or aqueous carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through ...
occurs, is buried in marine sediments. It is then implied that since there is a higher input of organic matter to the ocean than what is being buried, a large portion of it is used up or consumed within.
Fate of sinking organic carbon
Historically, sediments with the highest organic carbon contents were frequently found in areas with high surface water productivity or those with low bottom-water oxygen concentrations. 90% of organic carbon burial occurs in deposits of deltas andcontinental shelves
A continental shelf is a portion of a continent that is submerged under an area of relatively shallow water, known as a shelf sea. Much of these shelves were exposed by drops in sea level during glacial periods. The shelf surrounding an island ...
and upper slopes; this is due partly to short exposure time because of a shorter distance to the seafloor and the composition of the organic matter that is already deposited in those environments. Organic carbon burial is also sensitive to climate patterns: the accumulation rate of organic carbon was 50% larger during the glacial maximum compared to interglacial
An interglacial period (or alternatively interglacial, interglaciation) is a geological interval of warmer global average temperature lasting thousands of years that separates consecutive glacial periods within an ice age. The current Holocene i ...
s.
Degradation
POC is decomposed by a series of microbe-driven processes, such asmethanogenesis
Methanogenesis or biomethanation is the formation of methane coupled to energy conservation by microbes known as methanogens. Organisms capable of producing methane for energy conservation have been identified only from the domain Archaea, a group ...
and sulfate reduction, before burial in the seafloor. Degradation of POC also results in microbial methane production which is the main gas hydrate on the continental margins. Lignin and pollen are inherently resistant to degradation, and some studies show that inorganic matrices may also protect organic matter. Preservation rates of organic matter depend on other interdependent variables that vary nonlinearly in time and space. Although organic matter breakdown occurs rapidly in the presence of oxygen, microbes utilizing a variety of chemical species (via redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
gradients) can degrade organic matter in anoxic
The term anoxia means a total depletion in the level of oxygen, an extreme form of hypoxia or "low oxygen". The terms anoxia and hypoxia are used in various contexts:
* Anoxic waters, sea water, fresh water or groundwater that are depleted of diss ...
sediments. The burial depth at which degradation halts depends upon the sedimentation rate, the relative abundance of organic matter in the sediment, the type of organic matter being buried, and innumerable other variables. While decomposition of organic matter can occur in anoxic sediments when bacteria use oxidants other than oxygen (nitrate
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insolu ...
, sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
, Fe3+), decomposition tends to end short of complete mineralization
Mineralization may refer to:
* Mineralization (biology), when an inorganic substance precipitates in an organic matrix
** Biomineralization, a form of mineralization
** Mineralization of bone, an example of mineralization
** Mineralized tissues ar ...
. This occurs because of preferential decomposition of labile molecules over refractile molecules.
Burial
Organic carbon burial is an input of energy for underground biological environments and can regulate oxygen in the atmosphere at long time-scales (> 10,000 years). Burial can only take place if organic carbon arrives to the sea floor, making continental shelves and coastal margins the main storage of organic carbon from terrestrial and oceanic primary production.Fjord
In physical geography, a fjord or fiord () is a long, narrow inlet with steep sides or cliffs, created by a glacier. Fjords exist on the coasts of Alaska, Antarctica, British Columbia, Chile, Denmark, Germany, Greenland, the Faroe Islands, Icel ...
s, or cliffs created by glacial erosion, have also been identified as areas of significant carbon burial, with rates one hundred times greater than the ocean average. Particulate organic carbon is buried in oceanic sediments, creating a pathway between a rapidly available carbon pool in the ocean to its storage for geological timescales. Once carbon is sequestered in the seafloor, it is considered blue carbon
Blue Carbon refers to organic carbon that is captured and stored by the world's oceanic and coastal ecosystems, mostly by algae, seagrasses, macroalgae, mangroves, salt marshes and other plants in coastal wetlands. The term Blue Carbon was coine ...
. Burial rates can be calculated as the difference between the rate at which organic matter sinks and the rate at which it decomposes.
Calcium carbonate preservation
The precipitation of calcium carbonate is important as it results in a loss of alkalinity as well as a release of CO2 (Equation 4), and therefore a change in the rate of preservation of calcium carbonate can alter the partial pressure of CO2 in Earth's atmosphere. CaCO3 is supersatured in the great majority of ocean surface waters and undersaturated at depth, meaning the shells are more likely to dissolve as they sink to ocean depths. CaCO3 can also be dissolved through metabolic dissolution (i.e. can be used as food and excreted) and thus deep ocean sediments have very little calcium carbonate. The precipitation and burial of calcium carbonate in the ocean removes particulate inorganic carbon from the ocean and ultimately formslimestone
Limestone ( calcium carbonate ) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the minerals calcite and aragonite, which are different crystal forms of . Limestone forms w ...
. On time scales greater than 500,000 years Earth's climate is moderated by the flux of carbon in and out of the lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the portion of the upper mantle that behaves elastically on time scales of up to thousands of years ...
. Rocks formed in the ocean seafloor are recycled through plate tectonics
Plate tectonics (from the la, label= Late Latin, tectonicus, from the grc, τεκτονικός, lit=pertaining to building) is the generally accepted scientific theory that considers the Earth's lithosphere to comprise a number of larg ...
back to the surface and weathered or subducted into the mantle
A mantle is a piece of clothing, a type of cloak. Several other meanings are derived from that.
Mantle may refer to:
*Mantle (clothing), a cloak-like garment worn mainly by women as fashionable outerwear
**Mantle (vesture), an Eastern Orthodox ve ...
, the carbon outgassed by volcano
A volcano is a rupture in the crust of a planetary-mass object, such as Earth, that allows hot lava, volcanic ash, and gases to escape from a magma chamber below the surface.
On Earth, volcanoes are most often found where tectonic plates ...
es.
Human impacts
Oceans take up 15 – 40% of anthropogenic CO2, and so far roughly 40% of the carbon fromfossil fuel
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels ma ...
combustion has been taken up into the oceans. Because the Revelle factor increases with increasing CO2, a smaller fraction of the anthropogenic flux will be taken up by the ocean in the future. Current annual increase in atmospheric CO2 is approximately 4 gigatons of carbon. This induces climate change that drives carbon concentration and carbon-climate feedback processes that modifies ocean circulation and the physical and chemical properties of seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appro ...
, which alters CO2 uptake. Overfishing
Overfishing is the removal of a species of fish (i.e. fishing) from a body of water at a rate greater than that the species can replenish its population naturally (i.e. the overexploitation of the fishery's existing fish stock), resulting in t ...
and the plastic pollution
Plastic pollution is the accumulation of plastic objects and particles (e.g. plastic bottles, bags and microbeads) in the Earth's environment that adversely affects humans, wildlife and their habitat. Plastics that act as pollutants are cate ...
of the oceans contribute to the degraded state of the world's biggest carbon sink.
Ocean acidification
''Full article:Ocean acidification
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxid ...
''
The pH of the oceans is declining due to uptake of atmospheric CO2. The rise in dissolved carbon dioxide reduces the availability of the carbonate ion, reducing CaCO3 saturation state, thus making it thermodynamically harder to make CaCO3 shell. Carbonate ions preferentially bind to hydrogen ions to form bicarbonate, thus a reduction in carbonate ion availability increases the amount of unbound hydrogen ions, and decreases the amount of bicarbonate formed (Equations 1–3). pH is a measurement of hydrogen ion concentration, where a low pH means there are more unbound hydrogen ions. pH is therefore an indicator of carbonate speciation
Speciation is the evolutionary process by which populations evolve to become distinct species. The biologist Orator F. Cook coined the term in 1906 for cladogenesis, the splitting of lineages, as opposed to anagenesis, phyletic evolution withi ...
(the ''format'' of carbon present) in the oceans and can be used to assess how ''healthy'' the ocean is.
The list of organisms that may struggle due to ocean acidification include coccolithophore
Coccolithophores, or coccolithophorids, are single celled organisms which are part of the phytoplankton, the autotrophic (self-feeding) component of the plankton community. They form a group of about 200 species, and belong either to the king ...
s and foraminifera
Foraminifera (; Latin for "hole bearers"; informally called "forams") are single-celled organisms, members of a phylum or class of amoeboid protists characterized by streaming granular ectoplasm for catching food and other uses; and commonly ...
(the base of the marine food chain
Compared to terrestrial environments, marine environments have biomass pyramids which are inverted at the base. In particular, the biomass of consumers (copepods, krill, shrimp, forage fish) is larger than the biomass of primary producers. This ...
in many areas), human food sources such as oyster
Oyster is the common name for a number of different families of salt-water bivalve molluscs that live in marine or brackish habitats. In some species, the valves are highly calcified, and many are somewhat irregular in shape. Many, but not ...
s and mussel
Mussel () is the common name used for members of several families of bivalve molluscs, from saltwater and freshwater habitats. These groups have in common a shell whose outline is elongated and asymmetrical compared with other edible clams, which ...
s, and perhaps the most conspicuous, a structure built by organisms – the coral reefs. Most surface water will remain supersaturated with respect to CaCO3 (both calcite and aragonite) for some time on current emissions trajectories, but the organisms that require carbonate will likely be replaced in many areas. Coral reefs are under pressure from overfishing, nitrate pollution, and warming waters; ocean acidification will add additional stress on these important structures.
Iron fertilization
''Full article:Iron Fertilization
Iron fertilization is the intentional introduction of iron to iron-poor areas of the ocean surface to stimulate phytoplankton production. This is intended to enhance biological productivity and/or accelerate carbon dioxide () sequestration fro ...
''
Iron fertilization is a facet of geoengineering, which purposefully manipulates the Earth's climate system, typically in aspects of the carbon cycle or radiative forcing. Of current geoengineering interest is the possibility of accelerating the biological pump to increase export of carbon from the surface ocean. This increased export could theoretically remove excess carbon dioxide from the atmosphere for storage in the deep ocean. Ongoing investigations regarding artificial fertilization exist. Due to the scale of the ocean and the fast response times of heterotrophic communities to increases in primary production, it is difficult to determine whether limiting-nutrient fertilization results in an increase in carbon export. However, the majority of the community does not believe this is a reasonable or viable approach.
Dams and reservoirs
There are over 16 million dams in the world that alter carbon transport from rivers to oceans. Using data from the Global Reservoirs and Dams database, which contains approximately 7000 reservoirs that hold 77% of the total volume of water held back by dams (8000 km3), it is estimated that the delivery of carbon to the ocean has decreased by 13% since 1970 and is projected to reach 19% by 2030. The excess carbon contained in the reservoirs may emit an additional ~0.184 Gt of carbon to the atmosphere per year and an additional ~0.2 GtC will be buried in sediment. Prior to 2000, theMississippi
Mississippi () is a state in the Southeastern region of the United States, bordered to the north by Tennessee; to the east by Alabama; to the south by the Gulf of Mexico; to the southwest by Louisiana; and to the northwest by Arkansas. Miss ...
, the Niger
)
, official_languages =
, languages_type = National languagesGanges River
The Ganges ( ) (in India: Ganga ( ); in Bangladesh: Padma ( )). "The Ganges Basin, known in India as the Ganga and in Bangladesh as the Padma, is an international river to which India, Bangladesh, Nepal and China are the riparian states." is ...
basins account for 25 – 31% of all reservoir carbon burial. After 2000, the Paraná (home to 70 dams) and the Zambezi
The Zambezi River (also spelled Zambeze and Zambesi) is the fourth-longest river in Africa, the longest east-flowing river in Africa and the largest flowing into the Indian Ocean from Africa. Its drainage basin covers , slightly less than ha ...
(home to the largest reservoir) River basins exceeded the burial by the Mississippi. Other large contributors to carbon burial caused by damming occur on the Danube
The Danube ( ; ) is a river that was once a long-standing frontier of the Roman Empire and today connects 10 European countries, running through their territories or being a border. Originating in Germany, the Danube flows southeast for , pa ...
, the Amazon
Amazon most often refers to:
* Amazons, a tribe of female warriors in Greek mythology
* Amazon rainforest, a rainforest covering most of the Amazon basin
* Amazon River, in South America
* Amazon (company), an American multinational technolog ...
, the Yangtze
The Yangtze or Yangzi ( or ; ) is the longest river in Asia, the third-longest in the world, and the longest in the world to flow entirely within one country. It rises at Jari Hill in the Tanggula Mountains (Tibetan Plateau) and flows ...
, the Mekong
The Mekong or Mekong River is a trans-boundary river in East Asia and Southeast Asia. It is the world's twelfth longest river and the third longest in Asia. Its estimated length is , and it drains an area of , discharging of water annual ...
, the Yenisei
The Yenisey (russian: Енисе́й, ''Yeniséy''; mn, Горлог мөрөн, ''Gorlog mörön''; Buryat: Горлог мүрэн, ''Gorlog müren''; Tuvan: Улуг-Хем, ''Uluğ-Hem''; Khakas: Ким суғ, ''Kim suğ''; Ket: Ӄук, ...
, and the Tocantins
Tocantins () is one of the 26 states of Brazil. It is the newest state, formed in 1988 and encompassing what had formerly been the northern two-fifths of the state of Goiás. Tocantins covers and had an estimated population of 1,496,880 in 20 ...
Rivers.
Recent measurements
A 2020 study in Nature Communications, led by the University of Exeter, finds significantly higher net flux of carbon into the oceans compared to previous studies. The new study uses satellite data to account for small temperature differences between the surface of the ocean and the depth of a few meters where the measurements are made.Creative Commons Attribution 4.0 International License
This may be beneficial in terms of
climate change mitigation
Climate change mitigation is action to limit climate change by reducing emissions of greenhouse gases or removing those gases from the atmosphere. The recent rise in global average temperature is mostly caused by emissions from fossil fuels bu ...
but problematic in terms of ocean acidification
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxid ...
.
See also
* Phosphorus cycleReferences
External links
Current global map of the partial pressure of carbon dioxide at the ocean surface
{{biogeochemical cycle Carbon cycle Chemical oceanography