Ocean Chemistry on:
[Wikipedia]
[Google]
[Amazon]
Marine chemistry, also known as ocean chemistry or chemical oceanography, is the study of the chemical composition and processes of the world’s oceans, including the interactions between seawater, the atmosphere, the seafloor, and marine organisms. This field encompasses a wide range of topics, such as the cycling of elements like carbon, nitrogen, and phosphorus, the behavior of trace metals, and the study of gases and nutrients in marine environments. Marine chemistry plays a crucial role in understanding global
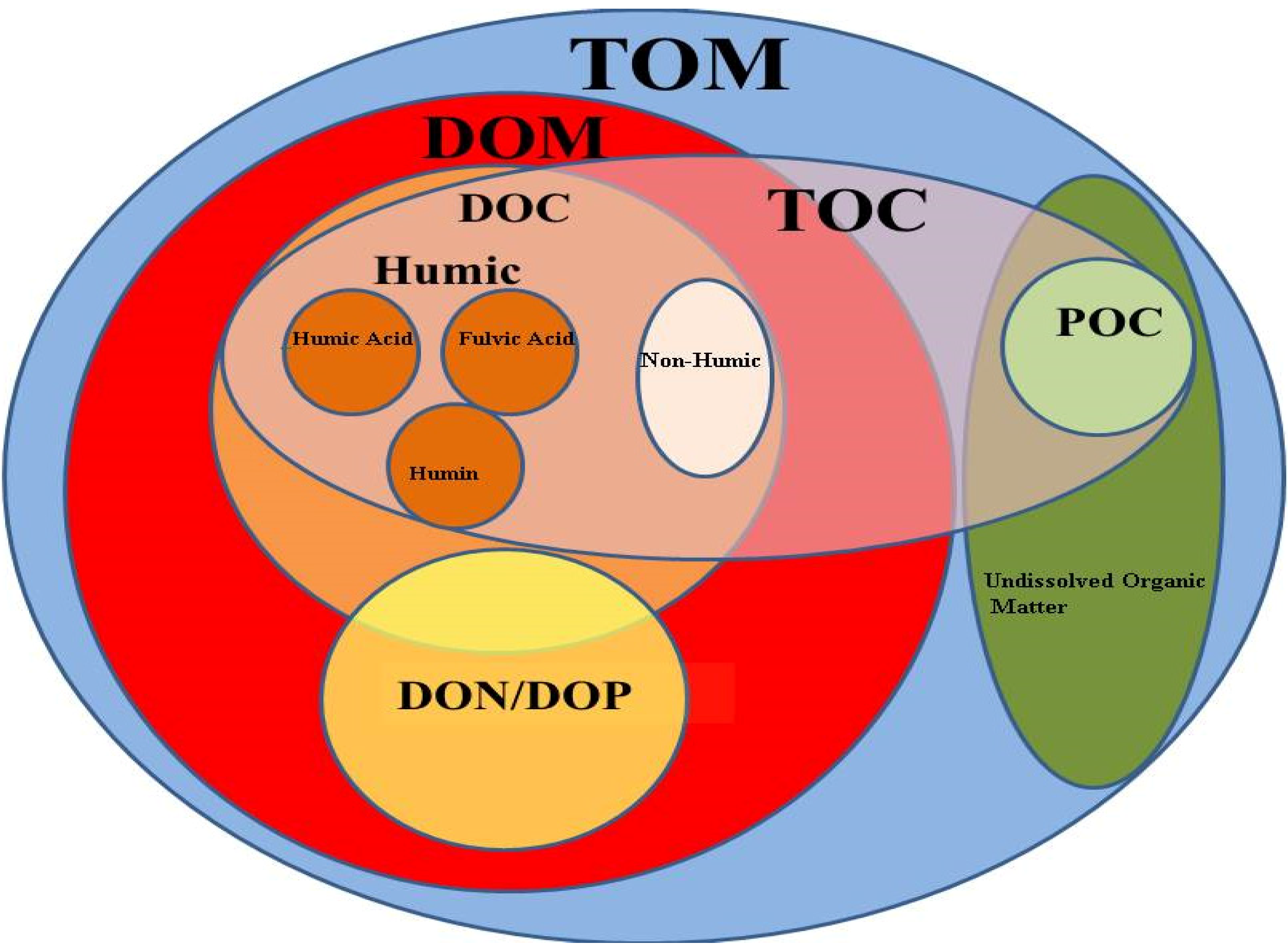 DOM is a critical component of the ocean's carbon pool and includes many molecules such as amino acids, sugars, and lipids. It represents about 90% of the total organic carbon in marine environments. Colored dissolved organic matter (CDOM) is estimated to range from 20-70% of the carbon content of the oceans, being higher near river outlets and lower in the open ocean. DOM can be recycled and put back into the food web through a process called microbial loop which is essential for nutrient cycling and supporting primary productivity. It also plays a vital role in the global regulation of oceanic carbon storage, as some forms resist microbial degradation and may exist within the ocean for centuries. Marine life is similar mainly in biochemistry to terrestrial organisms, and is the most prolific source of halogenated organic compounds.
DOM is a critical component of the ocean's carbon pool and includes many molecules such as amino acids, sugars, and lipids. It represents about 90% of the total organic carbon in marine environments. Colored dissolved organic matter (CDOM) is estimated to range from 20-70% of the carbon content of the oceans, being higher near river outlets and lower in the open ocean. DOM can be recycled and put back into the food web through a process called microbial loop which is essential for nutrient cycling and supporting primary productivity. It also plays a vital role in the global regulation of oceanic carbon storage, as some forms resist microbial degradation and may exist within the ocean for centuries. Marine life is similar mainly in biochemistry to terrestrial organisms, and is the most prolific source of halogenated organic compounds.
 In hydrothermal vents and similar environments, many extremophiles acquire energy through
In hydrothermal vents and similar environments, many extremophiles acquire energy through
 Seafloor spreading on
Seafloor spreading on
 Advanced analytical equipment such as mass spectrometers and chromatographs are applied to detect trace elements, isotopes, and organic compounds. This allows for precisely measuring nutrients, gases, and pollutants in marine environments. In recent years,
Advanced analytical equipment such as mass spectrometers and chromatographs are applied to detect trace elements, isotopes, and organic compounds. This allows for precisely measuring nutrients, gases, and pollutants in marine environments. In recent years,
biogeochemical cycle
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cyc ...
s, ocean circulation
An ocean current is a continuous, directed movement of seawater generated by a number of forces acting upon the water, including wind, the Coriolis effect, breaking waves, cabbeling, and temperature and salinity differences. Depth contours, ...
, and the effects of human activities, such as pollution and climate change, on oceanic systems. It is influenced by plate tectonics
Plate tectonics (, ) is the scientific theory that the Earth's lithosphere comprises a number of large tectonic plates, which have been slowly moving since 3–4 billion years ago. The model builds on the concept of , an idea developed durin ...
and seafloor spreading
Seafloor spreading, or seafloor spread, is a process that occurs at mid-ocean ridges, where new oceanic crust is formed through volcanic activity and then gradually moves away from the ridge.
History of study
Earlier theories by Alfred Wegener ...
, turbidity
Turbidity is the cloudiness or haziness of a fluid caused by large numbers of individual particles that are generally invisible to the naked eye, similar to smoke in air. The measurement of turbidity is a key test of both water clarity and wa ...
, currents
Currents, Current or The Current may refer to:
Science and technology
* Current (fluid), the flow of a liquid or a gas
** Air current, a flow of air
** Ocean current, a current in the ocean
*** Rip current, a kind of water current
** Current (hy ...
, sediment
Sediment is a solid material that is transported to a new location where it is deposited. It occurs naturally and, through the processes of weathering and erosion, is broken down and subsequently sediment transport, transported by the action of ...
s, pH levels, atmospheric constituents, metamorphic activity, and ecology.
The impact of human activity on the chemistry of the Earth's oceans has increased over time, with pollution from industry and various land-use practices significantly affecting the oceans. Moreover, increasing levels of carbon dioxide in the Earth's atmosphere have led to ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
, which has negative effects on marine ecosystems. The international community has agreed that restoring the chemistry of the oceans is a priority, and efforts toward this goal are tracked as part of Sustainable Development Goal 14
Sustainable Development Goal 14 (Goal 14 or SDG 14) is about "Life below water" and is one of the 17 Sustainable Development Goals established by the United Nations in 2015. The official wording is to "Conserve and sustainably use the oceans, seas ...
.
Due to the interrelatedness of the ocean, chemical oceanographers frequently work on problems relevant to physical oceanography
Physical oceanography is the study of physical conditions and physical processes within the ocean, especially the motions and physical properties of ocean waters.
Physical oceanography is one of several sub-domains into which oceanography is div ...
, geology
Geology (). is a branch of natural science concerned with the Earth and other astronomical objects, the rocks of which they are composed, and the processes by which they change over time. Modern geology significantly overlaps all other Earth ...
and geochemistry
Geochemistry is the science that uses the tools and principles of chemistry to explain the mechanisms behind major geological systems such as the Earth's crust and its oceans. The realm of geochemistry extends beyond the Earth, encompassing the e ...
, biology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and ...
and biochemistry
Biochemistry, or biological chemistry, is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology, a ...
, and atmospheric science
Atmospheric science is the study of the Atmosphere of Earth, Earth's atmosphere and its various inner-working physical processes. Meteorology includes atmospheric chemistry and atmospheric physics with a major focus on weather forecasting. Clima ...
. Many of them are investigating biogeochemical cycle
A biogeochemical cycle, or more generally a cycle of matter, is the movement and transformation of chemical elements and compounds between living organisms, the atmosphere, and the Earth's crust. Major biogeochemical cycles include the carbon cyc ...
s, and the marine carbon cycle
The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the Seabed, seafloor. The carbon cycle is a result of ma ...
in particular attracts significant interest due to its role in carbon sequestration
Carbon sequestration is the process of storing carbon in a carbon pool. It plays a crucial role in Climate change mitigation, limiting climate change by reducing the amount of Carbon dioxide in Earth's atmosphere, carbon dioxide in the atmosphe ...
and ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
. Other major topics of interest include analytical chemistry
Analytical skill, Analytical chemistry studies and uses instruments and methods to Separation process, separate, identify, and Quantification (science), quantify matter. In practice, separation, identification or quantification may constitute t ...
of the oceans, marine pollution
Marine pollution occurs when substances used or spread by humans, such as industrial waste, industrial, agricultural pollution, agricultural, and municipal solid waste, residential waste; particle (ecology), particles; noise; excess carbon dioxi ...
, and anthropogenic climate change
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
.
Organic compounds in the oceans
Dissolved Organic Matter (DOM)
 DOM is a critical component of the ocean's carbon pool and includes many molecules such as amino acids, sugars, and lipids. It represents about 90% of the total organic carbon in marine environments. Colored dissolved organic matter (CDOM) is estimated to range from 20-70% of the carbon content of the oceans, being higher near river outlets and lower in the open ocean. DOM can be recycled and put back into the food web through a process called microbial loop which is essential for nutrient cycling and supporting primary productivity. It also plays a vital role in the global regulation of oceanic carbon storage, as some forms resist microbial degradation and may exist within the ocean for centuries. Marine life is similar mainly in biochemistry to terrestrial organisms, and is the most prolific source of halogenated organic compounds.
DOM is a critical component of the ocean's carbon pool and includes many molecules such as amino acids, sugars, and lipids. It represents about 90% of the total organic carbon in marine environments. Colored dissolved organic matter (CDOM) is estimated to range from 20-70% of the carbon content of the oceans, being higher near river outlets and lower in the open ocean. DOM can be recycled and put back into the food web through a process called microbial loop which is essential for nutrient cycling and supporting primary productivity. It also plays a vital role in the global regulation of oceanic carbon storage, as some forms resist microbial degradation and may exist within the ocean for centuries. Marine life is similar mainly in biochemistry to terrestrial organisms, and is the most prolific source of halogenated organic compounds.
Particulate Organic Matter (POM)
POM includes of large organic particles, such as organisms, fecal pellets, and detritus, which settle through the water column. It is a major component of the biological pump, a process by which carbon is transferred from the surface ocean to the deep sea. As POM sinks, it decomposes by bacterial activity, releasing nutrients and carbon dioxide. The refractory POM fraction can settle on the ocean floor and make relevant contributions to carbon sequestration over a very long period of timeChemical ecology of extremophiles
The ocean is home to a variety of marine organisms known asextremophile
An extremophile () is an organism that is able to live (or in some cases thrive) in extreme environments, i.e., environments with conditions approaching or stretching the limits of what known life can adapt to, such as extreme temperature, press ...
s – organisms that thrive in extreme conditions of temperature, pressure, and light availability. Extremophiles inhabit many unique habitats in the ocean, such as hydrothermal vent
Hydrothermal vents are fissures on the seabed from which geothermally heated water discharges. They are commonly found near volcanically active places, areas where tectonic plates are moving apart at mid-ocean ridges, ocean basins, and hot ...
s, black smokers, cold seep
A cold seep (sometimes called a cold vent) is an area of the ocean floor where seepage of fluids rich in hydrogen sulfide, methane, and other hydrocarbons occurs, often in the form of a brine pool. ''Cold'' does not mean that the temperature ...
s, hypersaline regions, and sea ice brine pockets. Some scientists have speculated that life may have evolved from hydrothermal vents in the ocean.chemoautotroph
A chemotroph is an organism that obtains energy by the oxidation of electron donors in their environments. These molecules can be organic (chemoorganotrophs) or inorganic (chemolithotrophs). The chemotroph designation is in contrast to phototroph ...
y, using chemical compounds as energy sources, rather than light as in photoautotrophy. Hydrothermal vents enrich the nearby environment in chemicals such as elemental sulfur, H2, H2S, Fe2+, and methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
. Chemoautotrophic organisms, primarily prokaryotes, derive energy from these chemicals through redox reactions
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
. These organisms then serve as food sources for higher trophic level
The trophic level of an organism is the position it occupies in a food web. Within a food web, a food chain is a succession of organisms that eat other organisms and may, in turn, be eaten themselves. The trophic level of an organism is the ...
s, forming the basis of unique ecosystems.
Several different metabolisms are present in hydrothermal vent ecosystems. Many marine microorganisms, including ''Thiomicrospira'', ''Halothiobacillus'', and '' Beggiatoa'', are capable of oxidizing sulfur compounds, including elemental sulfur and the often toxic compound H2S. H2S is abundant in hydrothermal vents, formed through interactions between seawater and rock at the high temperatures found within vents. This compound is a major energy source, forming the basis of the sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
in hydrothermal vent ecosystems. In the colder waters surrounding vents, sulfur-oxidation can occur using oxygen as an electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. Electron acceptors are oxidizing agents.
The electron accepting power of an electron acceptor is measured by its redox potential.
In the ...
; closer to the vents, organisms must use alternate metabolic pathways or utilize another electron acceptor, such as nitrate. Some species of ''Thiomicrospira'' can utilize thiosulfate as an electron donor, producing elemental sulfur. Additionally, many marine microorganisms are capable of iron-oxidation, such as '' Mariprofundus ferrooxydans''. Iron-oxidation can be oxic, occurring in oxygen-rich parts of the ocean, or anoxic, requiring either an electron acceptor such as nitrate or light energy. In iron-oxidation, Fe(II) is used as an electron donor
In chemistry, an electron donor is a chemical entity that transfers electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process. An obsolete definition equated an electron dono ...
; conversely, iron-reducers utilize Fe(III) as an electron acceptor. These two metabolisms form the basis of the iron-redox cycle and may have contributed to banded iron formation
Banded iron formations (BIFs; also called banded ironstone formations) are distinctive units of sedimentary rock consisting of alternating layers of iron oxides and iron-poor chert. They can be up to several hundred meters in thickness and e ...
s.
At another extreme, some marine extremophiles inhabit sea ice brine pockets where temperature is very low and salinity is very high. Organisms trapped within freezing sea ice must adapt to a rapid change in salinity up to 3 times higher than that of regular seawater, as well as the rapid change to regular seawater salinity when ice melts. Most brine-pocket dwelling organisms are photosynthetic, therefore, these microenvironments can become hyperoxic, which can be toxic to its inhabitants. Thus, these extremophiles often produce high levels of antioxidants.
Plate tectonics
 Seafloor spreading on
Seafloor spreading on mid-ocean ridge
A mid-ocean ridge (MOR) is a undersea mountain range, seafloor mountain system formed by plate tectonics. It typically has a depth of about and rises about above the deepest portion of an ocean basin. This feature is where seafloor spreading ...
s is a global scale ion-exchange system. Hydrothermal vents at spreading centers introduce various amounts of iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
, manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
, silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and other elements into the ocean, some of which are recycled into the ocean crust
Oceanic crust is the uppermost layer of the oceanic portion of the Plate tectonics, tectonic plates. It is composed of the upper oceanic crust, with pillow lavas and a dike (geology), dike complex, and the lower oceanic crust, composed of troct ...
. Helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron. (In contrast, the most common isotope, helium-4, has two protons and two neutrons.) Helium-3 and hydrogen-1 are the only stable nuclides with ...
, an isotope that accompanies volcanism from the mantle, is emitted by hydrothermal vents and can be detected in plumes within the ocean.
Spreading rates on mid-ocean ridges vary between 10 and 200 mm/yr. Rapid spreading rates cause increased basalt
Basalt (; ) is an aphanite, aphanitic (fine-grained) extrusive igneous rock formed from the rapid cooling of low-viscosity lava rich in magnesium and iron (mafic lava) exposed at or very near the planetary surface, surface of a terrestrial ...
reactions with seawater. The magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
/calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
ratio will be lower because more magnesium ions are being removed from seawater and consumed by the rock, and more calcium ions are being removed from the rock and released to seawater. Hydrothermal activity at ridge crest is efficient in removing magnesium. A lower Mg/Ca ratio favors the precipitation of low-Mg calcite polymorphs of calcium carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skel ...
(calcite sea
A calcite sea is a sea in which low-magnesium calcite is the primary inorganic marine calcium carbonate precipitate. An aragonite sea is the alternate seawater chemistry in which aragonite and high-magnesium calcite are the primary inorganic carb ...
s).
Slow spreading at mid-ocean ridges has the opposite effect and will result in a higher Mg/Ca ratio favoring the precipitation of aragonite and high-Mg calcite polymorphs of calcium carbonate (aragonite sea
An aragonite sea contains aragonite and high-magnesium calcite as the primary inorganic calcium carbonate precipitates. The reason lies in the highly hydrated divalent ion, the second most abundant cation in seawater after , known to be a stron ...
s).
Experiments show that most modern high-Mg calcite organisms would have been low-Mg calcite in past calcite seas, meaning that the Mg/Ca ratio in an organism's skeleton varies with the Mg/Ca ratio of the seawater in which it was grown.
The mineralogy of reef-building and sediment-producing organisms is thus regulated by chemical reactions occurring along the mid-ocean ridge, the rate of which is controlled by the rate of sea-floor spreading.
Human impacts
Marine pollution
Climate change
Increasedcarbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
levels, mostly from burning fossil fuel
A fossil fuel is a flammable carbon compound- or hydrocarbon-containing material formed naturally in the Earth's crust from the buried remains of prehistoric organisms (animals, plants or microplanktons), a process that occurs within geolog ...
s, are changing ocean chemistry. Global warming
Present-day climate change includes both global warming—the ongoing increase in global average temperature—and its wider effects on Earth's climate system. Climate change in a broader sense also includes previous long-term changes ...
and changes in salinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensio ...
have significant implications for the ecology
Ecology () is the natural science of the relationships among living organisms and their Natural environment, environment. Ecology considers organisms at the individual, population, community (ecology), community, ecosystem, and biosphere lev ...
of marine environments.
Acidification
Deoxygenation
History
Early inquiries about marine chemistry usually concerned the origin ofsalinity
Salinity () is the saltiness or amount of salt (chemistry), salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensio ...
in the ocean, including work by Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, Alchemy, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the foun ...
. Modern chemical oceanography began as a field with the 1872–1876 ''Challenger'' expedition, led by the British Royal Navy which made the first systematic measurements of ocean chemistry. The chemical analysis of these samples providing the first systematic study of the composition of seawater was conducted by John Murray and George Forchhammer, leading to a better understanding of elements like chloride, sodium, and sulfate in ocean waters
The early 20th century saw significant advancements in marine chemistry, particularly with more accurate analytical techniques. Scientists like Martin Knudsen created the Knudsen Bottle, an instrument used to collect water samples from different ocean depths. Over the past three decades (1970s, 19802, and 1990s), a comprehensive evaluation of advancements in chemical oceanography was compiled through a National Science Foundation initiative known as Futures of Ocean Chemistry in the United States (FOCUS). This project brought together numerous prominent chemical oceanographers, marine chemists, and geochemists to contribute to the FOCUS report.
After World War II, advancements in geochemical techniques propelled marine chemistry into a new era. Researchers began using isotopic analysis to study ocean circulation and the carbon cycle. Roger Revelle and Hans Suess pioneered using radiocarbon dating to investigate oceanic carbon reservoirs and their exchange with the atmosphere.
Since the 1970s, the development of highly sophisticated instruments and computational models has revolutionized marine chemistry. Scientists can now measure trace metals, organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
s, and isotopic ratios with unprecedented precision. Studies of marine biogeochemical cycles, including the carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, and sulfur cycle
The sulfur cycle is a biogeochemical cycle in which the sulfur moves between rocks, waterways and living systems. It is important in geology as it affects many minerals and in life because sulfur is an essential element (CHNOPS), being a consti ...
s, have become an area of interest to understand global climate change. The use of remote sensing technology and global ocean observation programs, such as the International Geosphere-Biosphere Programme (IGBP), has provided large-scale data on ocean chemistry, allowing scientists to monitor ocean acidification
Ocean acidification is the ongoing decrease in the pH of the Earth's ocean. Between 1950 and 2020, the average pH of the ocean surface fell from approximately 8.15 to 8.05. Carbon dioxide emissions from human activities are the primary cause of ...
, deoxygenation, and other critical issues affecting the marine environment.
Tools used for analysis
Chemical oceanographers collect and measure chemicals in seawater, using the standard toolset ofanalytical chemistry
Analytical skill, Analytical chemistry studies and uses instruments and methods to Separation process, separate, identify, and Quantification (science), quantify matter. In practice, separation, identification or quantification may constitute t ...
as well as instruments like pH meter
A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH electro ...
s, electrical conductivity meters, fluorometers, and dissolved CO₂ meters. Most data are collected through shipboard measurements and from autonomous floats or buoys, but remote sensing
Remote sensing is the acquisition of information about an physical object, object or phenomenon without making physical contact with the object, in contrast to in situ or on-site observation. The term is applied especially to acquiring inform ...
is used as well. On an oceanographic research vessel, a CTD is used to measure electrical conductivity
Electrical resistivity (also called volume resistivity or specific electrical resistance) is a fundamental specific property of a material that measures its electrical resistance or how strongly it resists electric current. A low resistivity in ...
, temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
, and pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
, and is often mounted on a rosette of Nansen bottles to collect seawater for analysis. Sediments are commonly studied with a box corer or a sediment trap, and older sediments may be recovered by scientific drilling.
 Advanced analytical equipment such as mass spectrometers and chromatographs are applied to detect trace elements, isotopes, and organic compounds. This allows for precisely measuring nutrients, gases, and pollutants in marine environments. In recent years,
Advanced analytical equipment such as mass spectrometers and chromatographs are applied to detect trace elements, isotopes, and organic compounds. This allows for precisely measuring nutrients, gases, and pollutants in marine environments. In recent years, autonomous underwater vehicle
An autonomous underwater vehicle (AUV) is a robot that travels underwater without requiring continuous input from an operator. AUVs constitute part of a larger group of undersea systems known as unmanned underwater vehicles, a classification tha ...
s (AUVs) and remote sensing technology have enabled continuous, large-scale ocean chemistry monitoring, particularly for tracking changes in ocean acidification and nutrient cycles.
Marine chemistry on other planets and their moons
The chemistry of the subsurface ocean of Europa may be Earthlike. The subsurface ocean of Enceladus vents hydrogen and carbon dioxide to space.See also
* Global Ocean Data Analysis Project *Oceanography
Oceanography (), also known as oceanology, sea science, ocean science, and marine science, is the scientific study of the ocean, including its physics, chemistry, biology, and geology.
It is an Earth science, which covers a wide range of to ...
* Physical oceanography
Physical oceanography is the study of physical conditions and physical processes within the ocean, especially the motions and physical properties of ocean waters.
Physical oceanography is one of several sub-domains into which oceanography is div ...
* World Ocean Atlas
* Seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximat ...
* RISE project
References
{{Branches of chemistry Chemical oceanography Oceanographical terminology Geochemistry