Noble gas on:
[Wikipedia]
[Google]
[Amazon]
The noble gases (historically also the inert gases; sometimes referred to as aerogens) make up a class of
 Pierre Janssen and Joseph Norman Lockyer had discovered a new element on 18 August 1868 while looking at the
Pierre Janssen and Joseph Norman Lockyer had discovered a new element on 18 August 1868 while looking at the
 The noble gases are colorless, odorless, tasteless, and nonflammable under standard conditions. They were once labeled ''group 0'' in the periodic table because it was believed they had a valence of zero, meaning their
The noble gases are colorless, odorless, tasteless, and nonflammable under standard conditions. They were once labeled ''group 0'' in the periodic table because it was believed they had a valence of zero, meaning their
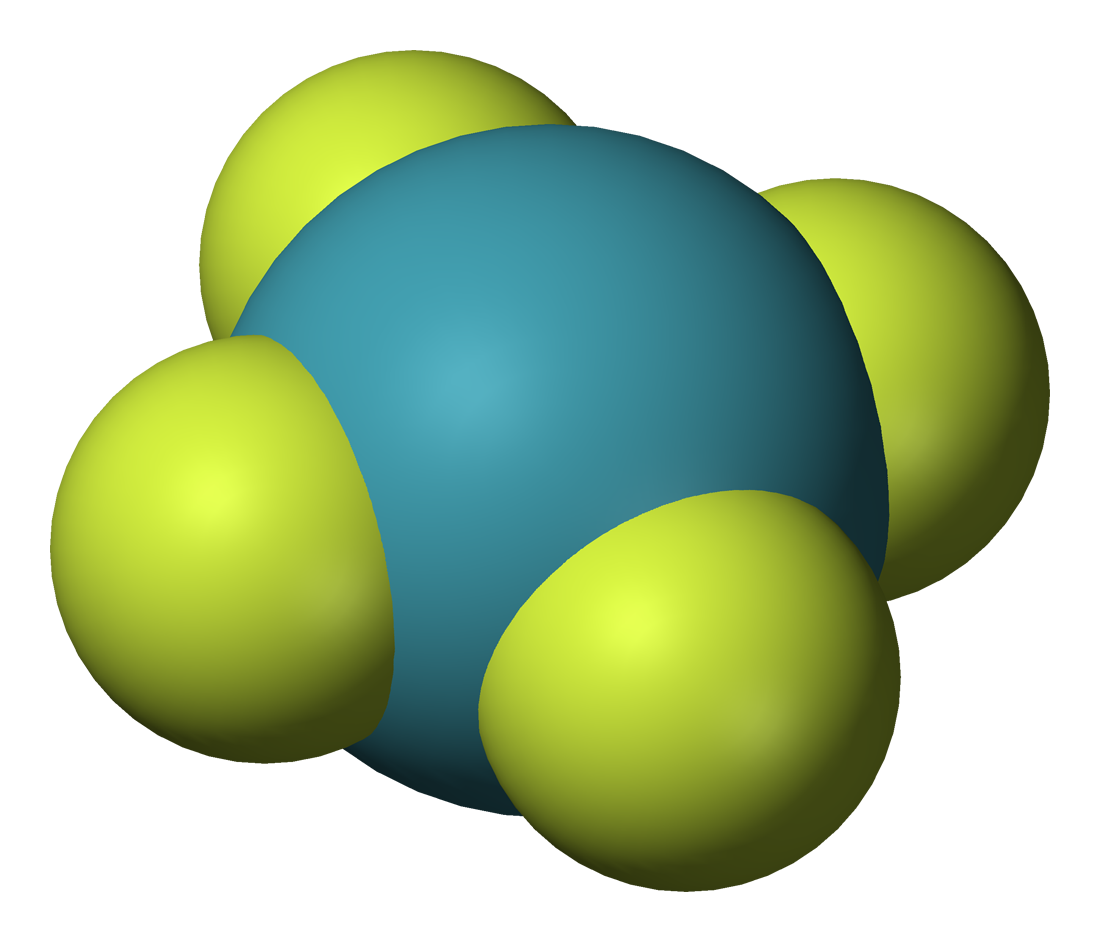 The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in chemical bonds have not been formed (although some helium-containing ions exist and there is some theoretical evidence for a few neutral helium-containing ones), while xenon, krypton, and argon have shown only minor reactivity. The reactivity follows the order Ne < He < Ar < Kr < Xe < Rn ≪ Og.
In 1933,
The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in chemical bonds have not been formed (although some helium-containing ions exist and there is some theoretical evidence for a few neutral helium-containing ones), while xenon, krypton, and argon have shown only minor reactivity. The reactivity follows the order Ne < He < Ar < Kr < Xe < Rn ≪ Og.
In 1933, 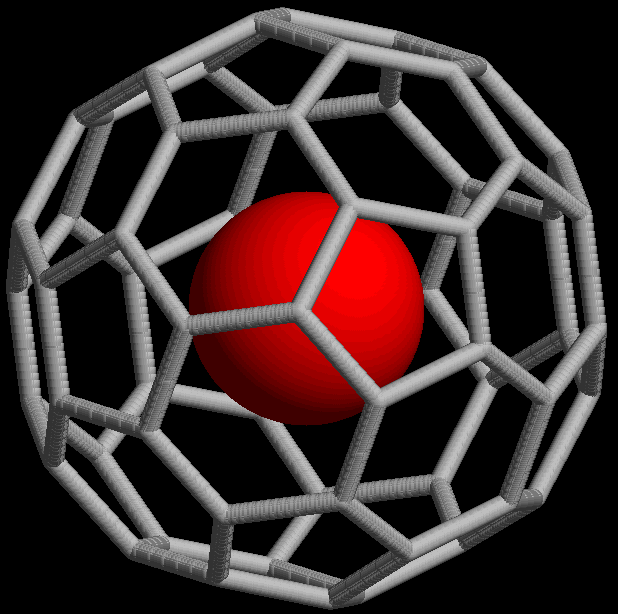 Noble gases can form
Noble gases can form  Noble gas compounds such as
Noble gas compounds such as
For large-scale use, helium is extracted by
 Noble gases have very low boiling and melting points, which makes them useful as
Noble gases have very low boiling and melting points, which makes them useful as  Since the ''Hindenburg'' disaster in 1937, helium has replaced hydrogen as a
Since the ''Hindenburg'' disaster in 1937, helium has replaced hydrogen as a  Noble gases are commonly used in
Noble gases are commonly used in
chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
s with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six naturally occurring noble gases are helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic ta ...
(He), neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypt ...
(Ne), argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice a ...
(Ar), krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is of ...
(Kr), xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
(Xe), and the radioactive radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains th ...
(Rn).
Oganesson (Og) is a synthetically produced highly radioactive element. Although IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
has used the term "noble gas" interchangeably with "group 18" and thus included oganesson, it may not be significantly chemically noble and is predicted to break the trend and be reactive due to relativistic effects
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of ...
. Because of the extremely short 0.7 ms half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of its only known isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
, its chemistry has not yet been investigated.
For the first six periods of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, the noble gases are exactly the members of group 18. Noble gases are typically highly unreactive except when under particular extreme conditions. The inertness of noble gases makes them very suitable in applications where reactions are not wanted. For example, argon is used in incandescent lamps to prevent the hot tungsten filament from oxidizing; also, helium is used in breathing gas by deep-sea divers to prevent oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
, nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
toxicity.
The properties of the noble gases can be well explained by modern theories of atomic structure: Their outer shell of valence electrons is considered to be "full", giving them little tendency to participate in chemical reactions, and it has been possible to prepare only a few hundred noble gas compounds. The melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which in ...
and boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding env ...
s for a given noble gas are close together, differing by less than ; that is, they are liquids over only a small temperature range.
Neon, argon, krypton, and xenon are obtained from air in an air separation
An air separation plant separates atmospheric air into its primary components, typically nitrogen and oxygen, and sometimes also argon and other rare inert gases.
The most common method for air separation is fractional distillation. Cryogenic a ...
unit using the methods of liquefaction of gases
Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The liquefaction of gases is a complicated process that uses various compressions and expansions to achieve high pressures and very low temperatures, using ...
and fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
. Helium is sourced from natural gas fields that have high concentrations of helium in the natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
, using cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
gas separation techniques, and radon is usually isolated from the radioactive decay
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
of dissolved radium
Radium is a chemical element with the symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rat ...
, thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
, or uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
compounds. Noble gases have several important applications in industries such as lighting, welding, and space exploration. A helium-oxygen breathing gas is often used by deep-sea divers at depths of seawater over . After the risks caused by the flammability of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
became apparent in the Hindenburg disaster
The ''Hindenburg'' disaster was an airship accident that occurred on May 6, 1937, in Manchester Township, New Jersey, United States. The German passenger airship LZ 129 ''Hindenburg'' caught fire and was destroyed during its attemp ...
, it was replaced with helium in blimps and balloons
A balloon is a flexible bag that can be inflated with a gas, such as helium, hydrogen, nitrous oxide, oxygen, and air. For special tasks, balloons can be filled with smoke, liquid water, granular media (e.g. sand, flour or rice), or light s ...
.
History
''Noble gas'' is translated from theGerman
German(s) may refer to:
* Germany (of or related to)
**Germania (historical use)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizens of Germany, see also German nationality law
**Ge ...
noun , first used in 1898 by Hugo Erdmann
Hugo Wilhelm Traugott Erdmann (8 May 1862 – 25 June 1910) was the German chemist who discovered, together with his doctoral advisor Jacob Volhard, the Volhard-Erdmann cyclization. In 1898 he was the first who coined the term ''noble gas'' (the ...
to indicate their extremely low level of reactivity. The name makes an analogy to the term " noble metals", which also have low reactivity. The noble gases have also been referred to as ''inert gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to ...
es'', but this label is deprecated as many noble gas compounds are now known. ''Rare gases'' is another term that was used, but this is also inaccurate because argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice a ...
forms a fairly considerable part (0.94% by volume, 1.3% by mass) of the Earth's atmosphere
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing fo ...
due to decay of radioactive potassium-40
Potassium-40 (40K) is a radioactive isotope of potassium which has a long half-life of 1.25 billion years. It makes up about 0.012% (120 ppm) of the total amount of potassium found in nature.
Potassium-40 undergoes three types of radioactive d ...
.
 Pierre Janssen and Joseph Norman Lockyer had discovered a new element on 18 August 1868 while looking at the
Pierre Janssen and Joseph Norman Lockyer had discovered a new element on 18 August 1868 while looking at the chromosphere
A chromosphere ("sphere of color") is the second layer of a star's atmosphere, located above the photosphere and below the solar transition region and corona. The term usually refers to the Sun's chromosphere, but not exclusively.
In the ...
of the Sun, and named it helium
Helium (from el, ἥλιος, helios, lit=sun) is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic ta ...
after the Greek word for the Sun, (). No chemical analysis was possible at the time, but helium was later found to be a noble gas. Before them, in 1784, the English chemist and physicist Henry Cavendish
Henry Cavendish ( ; 10 October 1731 – 24 February 1810) was an English natural philosopher and scientist who was an important experimental and theoretical chemist and physicist. He is noted for his discovery of hydrogen, which he termed "infl ...
had discovered that air contains a small proportion of a substance less reactive than nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
. A century later, in 1895, Lord Rayleigh
John William Strutt, 3rd Baron Rayleigh, (; 12 November 1842 – 30 June 1919) was an English mathematician and physicist who made extensive contributions to science. He spent all of his academic career at the University of Cambridge. A ...
discovered that samples of nitrogen from the air were of a different density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematicall ...
than nitrogen resulting from chemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking ...
s. Along with Scottish scientist William Ramsay at University College, London, Lord Rayleigh theorized that the nitrogen extracted from air was mixed with another gas, leading to an experiment that successfully isolated a new element, argon, from the Greek word (, "idle" or "lazy"). With this discovery, they realized an entire class of gases was missing from the periodic table. During his search for argon, Ramsay also managed to isolate helium for the first time while heating cleveite
Cleveite is an impure radioactive variety of uraninite containing uranium, found in Norway. It has the composition UO2 with about 10% of the uranium substituted by rare-earth elements. It was named after Swedish chemist Per Teodor Cleve.
Cleveit ...
, a mineral. In 1902, having accepted the evidence for the elements helium and argon, Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
included these noble gases as group 0 in his arrangement of the elements, which would later become the periodic table.
Ramsay continued his search for these gases using the method of fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
to separate liquid air into several components. In 1898, he discovered the elements krypton
Krypton (from grc, κρυπτός, translit=kryptos 'the hidden one') is a chemical element with the symbol Kr and atomic number 36. It is a colorless, odorless, tasteless noble gas that occurs in trace amounts in the atmosphere and is of ...
, neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypt ...
, and xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
, and named them after the Greek words (, "hidden"), (, "new"), and (, "stranger"), respectively. Radon
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colourless, odourless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains th ...
was first identified in 1898 by Friedrich Ernst Dorn, and was named ''radium emanation'', but was not considered a noble gas until 1904 when its characteristics were found to be similar to those of other noble gases. Rayleigh and Ramsay received the 1904 Nobel Prize
The Nobel Prizes ( ; sv, Nobelpriset ; no, Nobelprisen ) are five separate prizes that, according to Alfred Nobel's will of 1895, are awarded to "those who, during the preceding year, have conferred the greatest benefit to humankind." Alfr ...
s in Physics and in Chemistry, respectively, for their discovery of the noble gases; in the words of J. E. Cederblom, then president of the Royal Swedish Academy of Sciences
The Royal Swedish Academy of Sciences ( sv, Kungliga Vetenskapsakademien) is one of the royal academies of Sweden. Founded on 2 June 1739, it is an independent, non-governmental scientific organization that takes special responsibility for prom ...
, "the discovery of an entirely new group of elements, of which no single representative had been known with any certainty, is something utterly unique in the history of chemistry, being intrinsically an advance in science of peculiar significance".
The discovery of the noble gases aided in the development of a general understanding of atomic structure. In 1895, French chemist Henri Moissan attempted to form a reaction between fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactiv ...
, the most electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
element, and argon, one of the noble gases, but failed. Scientists were unable to prepare compounds of argon until the end of the 20th century, but these attempts helped to develop new theories of atomic structure. Learning from these experiments, Danish physicist Niels Bohr proposed in 1913 that the electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
s in atoms are arranged in shells surrounding the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
* Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
, and that for all noble gases except helium the outermost shell always contains eight electrons. In 1916, Gilbert N. Lewis
Gilbert Newton Lewis (October 23 or October 25, 1875 – March 23, 1946) was an American physical chemist and a Dean of the College of Chemistry at University of California, Berkeley. Lewis was best known for his discovery of the covalent bond a ...
formulated the '' octet rule'', which concluded an octet of electrons in the outer shell was the most stable arrangement for any atom; this arrangement caused them to be unreactive with other elements since they did not require any more electrons to complete their outer shell.
In 1962, Neil Bartlett discovered the first chemical compound of a noble gas, xenon hexafluoroplatinate. Compounds of other noble gases were discovered soon after: in 1962 for radon, radon difluoride (), which was identified by radiotracer techniques and in 1963 for krypton, krypton difluoride (). The first stable compound of argon was reported in 2000 when argon fluorohydride (HArF) was formed at a temperature of .
In October 2006, scientists from the Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory
Lawrence Livermore National Laboratory (LLNL) is a federal research facility in Livermore, California, United States. The lab was originally established as the University of California Radiation Laboratory, Livermore Branch in 1952 in response ...
successfully created synthetically oganesson, the seventh element in group 18, by bombarding californium
Californium is a radioactive chemical element with the symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory (then the University of California Radiation Laboratory), by bombarding c ...
with calcium.
Physical and atomic properties
The noble gases have weak interatomic force, and consequently have very lowmelting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which in ...
and boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding env ...
s. They are all monatomic gases under standard conditions, including the elements with larger atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&n ...
es than many normally solid elements. Helium has several unique qualities when compared with other elements: its boiling point at 1 atm is lower than those of any other known substance; it is the only element known to exhibit superfluidity; and, it is the only element that cannot be solidified by cooling at atmospheric pressure
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1013.25 millibars, ...
(an effect explained by quantum mechanics as its zero point energy
Zero-point energy (ZPE) is the lowest possible energy that a quantum mechanical system may have. Unlike in classical mechanics, quantum systems constantly fluctuate in their lowest energy state as described by the Heisenberg uncertainty ...
is too high to permit freezing) – a pressure of must be applied at a temperature of to convert it to a solid while a pressure of about 115 kbar is required at room temperature. The noble gases up to xenon have multiple stable isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s. Radon has no stable isotopes; its longest-lived isotope, 222Rn, has a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 3.8 days and decays to form helium and polonium, which ultimately decays to lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
. Melting and boiling points increase going down the group.
The noble gas atoms, like atoms in most groups, increase steadily in atomic radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ...
from one period to the next due to the increasing number of electrons. The size of the atom is related to several properties. For example, the ionization potential decreases with an increasing radius because the valence electrons in the larger noble gases are farther away from the nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
* Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucl ...
and are therefore not held as tightly together by the atom. Noble gases have the largest ionization potential among the elements of each period, which reflects the stability of their electron configuration and is related to their relative lack of chemical reactivity. Some of the heavier noble gases, however, have ionization potentials small enough to be comparable to those of other elements and molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and b ...
s. It was the insight that xenon has an ionization potential similar to that of the oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
molecule that led Bartlett to attempt oxidizing xenon using platinum hexafluoride, an oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
known to be strong enough to react with oxygen. Noble gases cannot accept an electron to form stable anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
s; that is, they have a negative electron affinity.
The macroscopic physical properties
A physical property is any property that is measurable, whose value describes a state of a physical system. The changes in the physical properties of a system can be used to describe its changes between momentary states. Physical properties are ...
of the noble gases are dominated by the weak van der Waals forces between the atoms. The attractive force increases with the size of the atom as a result of the increase in polarizability and the decrease in ionization potential. This results in systematic group trends: as one goes down group 18, the atomic radius, and with it the interatomic forces, increases, resulting in an increasing melting point, boiling point, enthalpy of vaporization, and solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
. The increase in density is due to the increase in atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&n ...
.
The noble gases are nearly ideal gases under standard conditions, but their deviations from the ideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first s ...
provided important clues for the study of intermolecular interactions. The Lennard-Jones potential
The Lennard-Jones potential (also termed the LJ potential or 12-6 potential) is an intermolecular pair potential. Out of all the intermolecular potentials, the Lennard-Jones potential is probably the one that has been the most extensively studie ...
, often used to model intermolecular interactions, was deduced in 1924 by John Lennard-Jones from experimental data on argon before the development of quantum mechanics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
provided the tools for understanding intermolecular forces from first principles. The theoretical analysis of these interactions became tractable because the noble gases are monatomic and the atoms spherical, which means that the interaction between the atoms is independent of direction, or isotropic
Isotropy is uniformity in all orientations; it is derived . Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also used to describ ...
.
Chemical properties
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, a ...
s cannot combine with those of other elements to form compounds. However, it was later discovered some do indeed form compounds, causing this label to fall into disuse.
Electron configuration
Like other groups, the members of this family show patterns in itselectron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
, especially the outermost shells resulting in trends in chemical behavior:
The noble gases have full valence electron shells
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" ( ...
. Valence electrons are the outermost electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
s of an atom and are normally the only electrons that participate in chemical bonding. Atoms with full valence electron shells are extremely stable and therefore do not tend to form chemical bonds and have little tendency to gain or lose electrons. However, heavier noble gases such as radon are held less firmly together by electromagnetic force than lighter noble gases such as helium, making it easier to remove outer electrons from heavy noble gases.
As a result of a full shell, the noble gases can be used in conjunction with the electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon ato ...
notation to form the ''noble gas notation''. To do this, the nearest noble gas that precedes the element in question is written first, and then the electron configuration is continued from that point forward. For example, the electron notation of
phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
is , while the noble gas notation is . This more compact notation makes it easier to identify elements, and is shorter than writing out the full notation of atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any ...
s.
The noble gases cross the boundary between blocks—helium is an s-element whereas the rest of members are p-elements—which is unusual among the IUPAC groups. Most, if not allIUPAC cannot decide on exact composition of group 3 which include elements from the f-block in some proposals. Their inclusion would make group 3 the second cross-block group. other IUPAC groups contain elements from ''one'' block each.
Compounds
 The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in chemical bonds have not been formed (although some helium-containing ions exist and there is some theoretical evidence for a few neutral helium-containing ones), while xenon, krypton, and argon have shown only minor reactivity. The reactivity follows the order Ne < He < Ar < Kr < Xe < Rn ≪ Og.
In 1933,
The noble gases show extremely low chemical reactivity; consequently, only a few hundred noble gas compounds have been formed. Neutral compounds in which helium and neon are involved in chemical bonds have not been formed (although some helium-containing ions exist and there is some theoretical evidence for a few neutral helium-containing ones), while xenon, krypton, and argon have shown only minor reactivity. The reactivity follows the order Ne < He < Ar < Kr < Xe < Rn ≪ Og.
In 1933, Linus Pauling
Linus Carl Pauling (; February 28, 1901August 19, 1994) was an American chemist, biochemist, chemical engineer, peace activist, author, and educator. He published more than 1,200 papers and books, of which about 850 dealt with scientific topi ...
predicted that the heavier noble gases could form compounds with fluorine and oxygen. He predicted the existence of krypton hexafluoride () and xenon hexafluoride (), speculated that might exist as an unstable compound, and suggested that xenic acid could form perxenate salts. These predictions were shown to be generally accurate, except that is now thought to be both thermodynamically and kinetically unstable.
Xenon compounds are the most numerous of the noble gas compounds that have been formed. Most of them have the xenon atom in the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of +2, +4, +6, or +8 bonded to highly electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
atoms such as fluorine or oxygen, as in xenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwis ...
(), xenon tetrafluoride
Xenon tetrafluoride is a chemical compound with chemical formula . It was the first discovered binary compound of a noble gas. It is produced by the chemical reaction of xenon with fluorine:
: Xe + 2 →
This reaction is exothermic, rel ...
(), xenon hexafluoride (), xenon tetroxide (), and sodium perxenate (). Xenon reacts with fluorine to form numerous xenon fluorides according to the following equations:
::Xe + F2 → XeF2
::Xe + 2F2 → XeF4
::Xe + 3F2 → XeF6
Some of these compounds have found use in chemical synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In mod ...
as oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
s; , in particular, is commercially available and can be used as a fluorinating agent. As of 2007, about five hundred compounds of xenon bonded to other elements have been identified, including organoxenon compounds (containing xenon bonded to carbon), and xenon bonded to nitrogen, chlorine, gold, mercury, and xenon itself. Compounds of xenon bound to boron, hydrogen, bromine, iodine, beryllium, sulphur, titanium, copper, and silver have also been observed but only at low temperatures in noble gas matrices, or in supersonic noble gas jets.
Radon is more reactive than xenon, and forms chemical bonds more easily than xenon does. However, due to the high radioactivity and short half-life of radon isotopes, only a few fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts ty ...
s and oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the E ...
s of radon have been formed in practice. Radon goes further towards metallic behavior than xenon; the difluoride RnF2 is highly ionic, and cationic Rn2+ is formed in halogen fluoride solutions. For this reason, kinetic hindrance makes it difficult to oxidize radon beyond the +2 state. Only tracer experiments appear to have succeeded in doing so, probably forming RnF4, RnF6, and RnO3.
Krypton is less reactive than xenon, but several compounds have been reported with krypton in the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of +2. Krypton difluoride is the most notable and easily characterized. Under extreme conditions, krypton reacts with fluorine to form KrF2 according to the following equation:
::Kr + F2 → KrF2
Compounds in which krypton forms a single bond to nitrogen and oxygen have also been characterized, but are only stable below and respectively.
Krypton atoms chemically bound to other nonmetals (hydrogen, chlorine, carbon) as well as some late transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s (copper, silver, gold) have also been observed, but only either at low temperatures in noble gas matrices, or in supersonic noble gas jets. Similar conditions were used to obtain the first few compounds of argon in 2000, such as argon fluorohydride (HArF), and some bound to the late transition metals copper, silver, and gold. As of 2007, no stable neutral molecules involving covalently bound helium or neon are known.
Extrapolation from periodic trends predict that oganesson should be the most reactive of the noble gases; more sophisticated theoretical treatments indicate greater reactivity than such extrapolations suggest, to the point where the applicability of the descriptor "noble gas" has been questioned. Oganesson is expected to be rather like silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
or tin in group 14: a reactive element with a common +4 and a less common +2 state, which at room temperature and pressure is not a gas but rather a solid semiconductor. Empirical / experimental testing will be required to validate these predictions. (On the other hand, flerovium
Flerovium is a superheavy chemical element with symbol Fl and atomic number 114. It is an extremely radioactive synthetic element. It is named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dub ...
, despite being in group 14, is predicted to be unusually volatile, which suggests noble gas-like properties.)
The noble gases—including helium—can form stable molecular ion
Mass spectral interpretation is the method employed to identify the chemical formula, characteristic fragment patterns and possible fragment ions from the mass spectra. Mass spectra is a plot of relative abundance against mass-to-charge ratio. It i ...
s in the gas phase. The simplest is the helium hydride molecular ion, HeH+, discovered in 1925. Because it is composed of the two most abundant elements in the universe, hydrogen and helium, it is believed to occur naturally in the interstellar medium
In astronomy, the interstellar medium is the matter and radiation that exist in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstella ...
, although it has not been detected yet. In addition to these ions, there are many known neutral excimers of the noble gases. These are compounds such as ArF and KrF that are stable only when in an excited electronic state; some of them find application in excimer laser
An excimer laser, sometimes more correctly called an exciplex laser, is a form of ultraviolet laser which is commonly used in the production of microelectronic devices, semiconductor based integrated circuits or "chips", eye surgery, and microm ...
s.
In addition to the compounds where a noble gas atom is involved in a covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between ato ...
, noble gases also form non-covalent
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. Th ...
compounds. The clathrate
A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word ''clathrate'' is derived from the Latin (), meaning ‘with bars, latticed’. Most clathrate compounds are polymeric and completely envelo ...
s, first described in 1949, consist of a noble gas atom trapped within cavities of crystal lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
s of certain organic and inorganic substances. The essential condition for their formation is that the guest (noble gas) atoms must be of appropriate size to fit in the cavities of the host crystal lattice. For instance, argon, krypton, and xenon form clathrates with hydroquinone
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups bonded to a benzene ring in a ''pa ...
, but helium and neon do not because they are too small or insufficiently polarizable to be retained. Neon, argon, krypton, and xenon also form clathrate hydrates, where the noble gas is trapped in ice.
 Noble gases can form
Noble gases can form endohedral fullerene
Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex called La@C60 was synthesized in 1985. The @ (at sign) in the ...
compounds, in which the noble gas atom is trapped inside a fullerene molecule. In 1993, it was discovered that when , a spherical molecule consisting of 60 carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
atoms, is exposed to noble gases at high pressure, complexes such as can be formed (the ''@'' notation indicates He is contained inside but not covalently bound to it). As of 2008, endohedral complexes with helium, neon, argon, krypton, and xenon have been created. These compounds have found use in the study of the structure and reactivity of fullerenes by means of the nuclear magnetic resonance of the noble gas atom.
xenon difluoride
Xenon difluoride is a powerful fluorinating agent with the chemical formula , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwis ...
() are considered to be hypervalent because they violate the octet rule. Bonding in such compounds can be explained using a three-center four-electron bond model. This model, first proposed in 1951, considers bonding of three collinear atoms. For example, bonding in is described by a set of three molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of find ...
s (MOs) derived from p-orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any ...
s on each atom. Bonding results from the combination of a filled p-orbital from Xe with one half-filled p-orbital from each F atom, resulting in a filled bonding orbital, a filled non-bonding orbital, and an empty antibonding
In chemical bonding theory, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more no ...
orbital. The highest occupied molecular orbital
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''fronti ...
is localized on the two terminal atoms. This represents a localization of charge that is facilitated by the high electronegativity of fluorine.
The chemistry of the heavier noble gases, krypton and xenon, are well established. The chemistry of the lighter ones, argon and helium, is still at an early stage, while a neon compound is yet to be identified.
Occurrence and production
The abundances of the noble gases in the universe decrease as theiratomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
s increase. Helium is the most common element in the universe
The universe is all of space and time and their contents, including planets, stars, galaxies, and all other forms of matter and energy. The Big Bang theory is the prevailing cosmological description of the development of the univers ...
after hydrogen, with a mass fraction of about 24%. Most of the helium in the universe was formed during Big Bang nucleosynthesis, but the amount of helium is steadily increasing due to the fusion of hydrogen in stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. A ...
(and, to a very slight degree, the alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
of heavy elements). Abundances on Earth follow different trends; for example, helium is only the third most abundant noble gas in the atmosphere. The reason is that there is no primordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before t ...
helium in the atmosphere; due to the small mass of the atom, helium cannot be retained by the Earth's gravitational field
In physics, a gravitational field is a model used to explain the influences that a massive body extends into the space around itself, producing a force on another massive body. Thus, a gravitational field is used to explain gravitational pheno ...
. Helium on Earth comes from the alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
of heavy elements such as uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
found in the Earth's crust, and tends to accumulate in natural gas deposits. The abundance of argon, on the other hand, is increased as a result of the beta decay
In nuclear physics, beta decay (β-decay) is a type of radioactive decay in which a beta particle (fast energetic electron or positron) is emitted from an atomic nucleus, transforming the original nuclide to an isobar of that nuclide. For ...
of potassium-40
Potassium-40 (40K) is a radioactive isotope of potassium which has a long half-life of 1.25 billion years. It makes up about 0.012% (120 ppm) of the total amount of potassium found in nature.
Potassium-40 undergoes three types of radioactive d ...
, also found in the Earth's crust, to form argon-40
Argon (18Ar) has 26 known isotopes, from 29Ar to 54Ar and 1 isomer (32mAr), of which three are stable (36Ar, 38Ar, and 40Ar). On the Earth, 40Ar makes up 99.6% of natural argon. The longest-lived radioactive isotopes are 39Ar with a half-life of ...
, which is the most abundant isotope of argon on Earth despite being relatively rare in the Solar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
. This process is the basis for the potassium-argon dating method. Xenon has an unexpectedly low abundance in the atmosphere, in what has been called the ''missing xenon problem''; one theory is that the missing xenon may be trapped in minerals inside the Earth's crust. After the discovery of xenon dioxide
Xenon dioxide, or xenon(IV) oxide, is a compound of xenon and oxygen with formula XeO2 which was synthesized in 2011. It is synthesized at 0 °C by hydrolysis of xenon tetrafluoride in aqueous sulfuric acid: XeF4 + 2H2O -> XeO2 + 4HF
Struc ...
, research showed that Xe can substitute for Si in quartz
Quartz is a hard, crystalline mineral composed of silica ( silicon dioxide). The atoms are linked in a continuous framework of SiO4 silicon-oxygen tetrahedra, with each oxygen being shared between two tetrahedra, giving an overall chemical f ...
. Radon is formed in the lithosphere
A lithosphere () is the rigid, outermost rocky shell of a terrestrial planet or natural satellite. On Earth, it is composed of the crust and the portion of the upper mantle that behaves elastically on time scales of up to thousands of years ...
by the alpha decay
Alpha decay or α-decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus) and thereby transforms or 'decays' into a different atomic nucleus, with a mass number that is reduced by four and an at ...
of radium. It can seep into buildings through cracks in their foundation and accumulate in areas that are not well ventilated. Due to its high radioactivity, radon presents a significant health hazard; it is implicated in an estimated 21,000 lung cancer
Lung cancer, also known as lung carcinoma (since about 98–99% of all lung cancers are carcinomas), is a malignant lung tumor characterized by uncontrolled cell growth in tissues of the lung. Lung carcinomas derive from transformed, mali ...
deaths per year in the United States alone. Oganesson does not occur in nature and is instead created manually by scientists.
fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
from natural gas, which can contain up to 7% helium.
Neon, argon, krypton, and xenon are obtained from air using the methods of liquefaction of gases
Liquefaction of gases is physical conversion of a gas into a liquid state (condensation). The liquefaction of gases is a complicated process that uses various compressions and expansions to achieve high pressures and very low temperatures, using ...
, to convert elements to a liquid state, and fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
, to separate mixtures into component parts. Helium is typically produced by separating it from natural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbon d ...
, and radon is isolated from the radioactive decay of radium compounds. The prices of the noble gases are influenced by their natural abundance, with argon being the cheapest and xenon the most expensive. As an example, the adjacent table lists the 2004 prices in the United States for laboratory quantities of each gas.
Applications
cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cr ...
refrigerant
A refrigerant is a working fluid used in the refrigeration cycle of air conditioning systems and heat pumps where in most cases they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated ...
s. In particular, liquid helium, which boils at , is used for superconducting magnet
A superconducting magnet is an electromagnet made from coils of superconducting wire. They must be cooled to cryogenic temperatures during operation. In its superconducting state the wire has no electrical resistance and therefore can conduct much ...
s, such as those needed in nuclear magnetic resonance imaging and nuclear magnetic resonance. Liquid neon, although it does not reach temperatures as low as liquid helium, also finds use in cryogenics because it has over 40 times more refrigerating capacity than liquid helium and over three times more than liquid hydrogen.
Helium is used as a component of breathing gases
A breathing gas is a mixture of gaseous chemical elements and compounds used for respiration. Air is the most common and only natural breathing gas, but other mixtures of gases, or pure oxygen, are also used in breathing equipment and enclosed h ...
to replace nitrogen, due its low solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
in fluids, especially in lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids in ...
. Gases are absorbed by the blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the cir ...
and body tissues when under pressure like in scuba diving
Scuba diving is a mode of underwater diving whereby divers use breathing equipment that is completely independent of a surface air supply. The name "scuba", an acronym for " Self-Contained Underwater Breathing Apparatus", was coined by Chr ...
, which causes an anesthetic effect known as nitrogen narcosis. Due to its reduced solubility, little helium is taken into cell membranes
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (t ...
, and when helium is used to replace part of the breathing mixtures, such as in trimix or heliox, a decrease in the narcotic effect of the gas at depth is obtained. Helium's reduced solubility offers further advantages for the condition known as decompression sickness, or ''the bends''. The reduced amount of dissolved gas in the body means that fewer gas bubbles form during the decrease in pressure of the ascent. Another noble gas, argon, is considered the best option for use as a drysuit
A dry suit or drysuit provides the wearer with environmental protection by way of thermal insulation and exclusion of water, and is worn by divers, boaters, water sports enthusiasts, and others who work or play in or near cold or contaminated ...
inflation gas for scuba diving. Helium is also used as filling gas in nuclear fuel rods for nuclear reactors.
 Since the ''Hindenburg'' disaster in 1937, helium has replaced hydrogen as a
Since the ''Hindenburg'' disaster in 1937, helium has replaced hydrogen as a lifting gas
A lifting gas or lighter-than-air gas is a gas that has a density lower than normal atmospheric gases and rises above them as a result. It is required for aerostats to create buoyancy, particularly in lighter-than-air aircraft, which include free ...
in blimps and balloon
A balloon is a flexible bag that can be inflated with a gas, such as helium, hydrogen, nitrous oxide, oxygen, and air. For special tasks, balloons can be filled with smoke, liquid water, granular media (e.g. sand, flour or rice), or lig ...
s due to its lightness and incombustibility, despite an 8.6% decrease in buoyancy.
In many applications, the noble gases are used to provide an inert atmosphere. Argon is used in the synthesis of air-sensitive compounds that are sensitive to nitrogen. Solid argon is also used for the study of very unstable compounds, such as reactive intermediate
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, s ...
s, by trapping them in an inert matrix
Matrix most commonly refers to:
* ''The Matrix'' (franchise), an American media franchise
** '' The Matrix'', a 1999 science-fiction action film
** "The Matrix", a fictional setting, a virtual reality environment, within ''The Matrix'' (franchi ...
at very low temperatures. Helium is used as the carrier medium in gas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substanc ...
, as a filler gas for thermometers
A thermometer is a device that measures temperature or a temperature gradient (the degree of hotness or coldness of an object). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a mercury-in-glass thermomete ...
, and in devices for measuring radiation, such as the Geiger counter
A Geiger counter (also known as a Geiger–Müller counter) is an electronic instrument used for detecting and measuring ionizing radiation. It is widely used in applications such as radiation dosimetry, radiological protection, experimental p ...
and the bubble chamber. Helium and argon are both commonly used to shield welding arc
Welding is a fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing fusion. Welding is distinct from lower temperature techniques such as braz ...
s and the surrounding base metal
A base metal is a common and inexpensive metal, as opposed to a precious metal such as gold or silver. In numismatics, coins often derived their value from the precious metal content; however, base metals have also been used in coins in the past ...
from the atmosphere during welding and cutting, as well as in other metallurgical processes and in the production of silicon for the semiconductor industry.
 Noble gases are commonly used in
Noble gases are commonly used in lighting
Lighting or illumination is the deliberate use of light to achieve practical or aesthetic effects. Lighting includes the use of both artificial light sources like lamps and light fixtures, as well as natural illumination by capturing dayl ...
because of their lack of chemical reactivity. Argon, mixed with nitrogen, is used as a filler gas for incandescent light bulb
An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a wire filament heated until it glows. The filament is enclosed in a glass bulb with a vacuum or inert gas to protect the filament from oxi ...
s. Krypton is used in high-performance light bulbs, which have higher color temperatures and greater efficiency, because it reduces the rate of evaporation of the filament more than argon; halogen lamps, in particular, use krypton mixed with small amounts of compounds of iodine
Iodine is a chemical element with the Symbol (chemistry), symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , ...
or bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
. The noble gases glow in distinctive colors when used inside gas-discharge lamp
Gas-discharge lamps are a family of artificial light sources that generate light by sending an electric discharge through an ionized gas, a plasma.
Typically, such lamps use a
noble gas (argon, neon, krypton, and xenon) or a mixture of t ...
s, such as " neon lights". These lights are called after neon but often contain other gases and phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or v ...
s, which add various hues to the orange-red color of neon. Xenon is commonly used in xenon arc lamp
A xenon arc lamp is a highly specialized type of gas discharge lamp, an electric light that produces light by passing electricity through ionized xenon gas at high pressure. It produces a bright white light to simulate sunlight, with applications ...
s, which, due to their nearly continuous spectrum
In physics, a continuous spectrum usually means a set of attainable values for some physical quantity (such as energy or wavelength) that is best described as an interval of real numbers, as opposed to a discrete spectrum, a set of attainable ...
that resembles daylight, find application in film projectors and as automobile headlamps.
The noble gases are used in excimer laser
An excimer laser, sometimes more correctly called an exciplex laser, is a form of ultraviolet laser which is commonly used in the production of microelectronic devices, semiconductor based integrated circuits or "chips", eye surgery, and microm ...
s, which are based on short-lived electronically excited molecules known as excimers. The excimers used for lasers may be noble gas dimers such as Ar2, Kr2 or Xe2, or more commonly, the noble gas is combined with a halogen in excimers such as ArF, KrF, XeF, or XeCl. These lasers produce ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation ...
light, which, due to its short wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
(193 nm for ArF and 248 nm for KrF), allows for high-precision imaging. Excimer lasers have many industrial, medical, and scientific applications. They are used for microlithography
Microlithography is a general name for any manufacturing process that can create a minutely patterned thin film of protective materials over a substrate, such as a silicon wafer, in order to protect selected areas of it during subsequent etchi ...
and microfabrication
Microfabrication is the process of fabricating miniature structures of micrometre scales and smaller. Historically, the earliest microfabrication processes were used for integrated circuit fabrication, also known as " semiconductor manufacturing ...
, which are essential for integrated circuit
An integrated circuit or monolithic integrated circuit (also referred to as an IC, a chip, or a microchip) is a set of electronic circuits on one small flat piece (or "chip") of semiconductor material, usually silicon. Large numbers of tiny ...
manufacture, and for laser surgery, including laser angioplasty and eye surgery
Eye surgery, also known as ophthalmic or ocular surgery, is surgery performed on the eye or its adnexa, by an ophthalmologist or sometimes, an optometrist. Eye surgery is synonymous with ophthalmology. The eye is a very fragile organ, and requ ...
.
Some noble gases have direct application in medicine. Helium is sometimes used to improve the ease of breathing of asthma
Asthma is a long-term inflammatory disease of the airways of the lungs. It is characterized by variable and recurring symptoms, reversible airflow obstruction, and easily triggered bronchospasms. Symptoms include episodes of wheezing, co ...
sufferers. Xenon is used as an anesthetic because of its high solubility in lipids, which makes it more potent than the usual nitrous oxide
Nitrous oxide (dinitrogen oxide or dinitrogen monoxide), commonly known as laughing gas, nitrous, or nos, is a chemical compound, an oxide of nitrogen with the formula . At room temperature, it is a colourless non-flammable gas, and ha ...
, and because it is readily eliminated from the body, resulting in faster recovery. Xenon finds application in medical imaging of the lungs through hyperpolarized MRI. Radon, which is highly radioactive and is only available in minute amounts, is used in radiotherapy
Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Rad ...
.
Noble gases, particularly xenon, are predominantly used in ion engines
An ion thruster, ion drive, or ion engine is a form of electric propulsion used for spacecraft propulsion. It creates thrust by accelerating ions using electricity.
An ion thruster ionizes a neutral gas by extracting some electrons out of a ...
due to their inertness. Since ion engines are not driven by chemical reactions, chemically inert fuels are desired to prevent unwanted reaction between the fuel and anything else on the engine.
Oganesson is too unstable to work with and has no known application other than research.
Discharge color
The color of gas discharge emission depends on several factors, including the following: * discharge parameters (local value ofcurrent density
In electromagnetism, current density is the amount of charge per unit time that flows through a unit area of a chosen cross section. The current density vector is defined as a vector whose magnitude is the electric current per cross-sectional a ...
and electric field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field ...
, temperature, etc. – note the color variation along the discharge in the top row);
* gas purity (even small fraction of certain gases can affect color);
* material of the discharge tube envelope – note suppression of the UV and blue components in the bottom-row tubes made of thick household glass.
See also
* Noble gas (data page), for extended tables of physical properties. * Noble metal, for metals that are resistant to corrosion or oxidation. *Inert gas
An inert gas is a gas that does not readily undergo chemical reactions with other chemical substances and therefore does not readily form chemical compounds. The noble gases often do not react with many substances and were historically referred to ...
, for any gas that is not reactive under normal circumstances.
*Industrial gas
Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also av ...
* Neutronium
* Octet rule
Notes
References
* * * * * * * * {{DEFAULTSORT:Noble Gas Groups (periodic table)