Neutron cross-section on:
[Wikipedia]
[Google]
[Amazon]
In
 For a given target and reaction, the cross section is strongly dependent on the neutron speed. In the extreme case, the cross section can be, at low energies, either zero (the energy for which the cross section becomes significant is called threshold energy) or much larger than at high energies.
Therefore, a cross section should be defined either at a given energy or should be averaged in an energy range (or group).
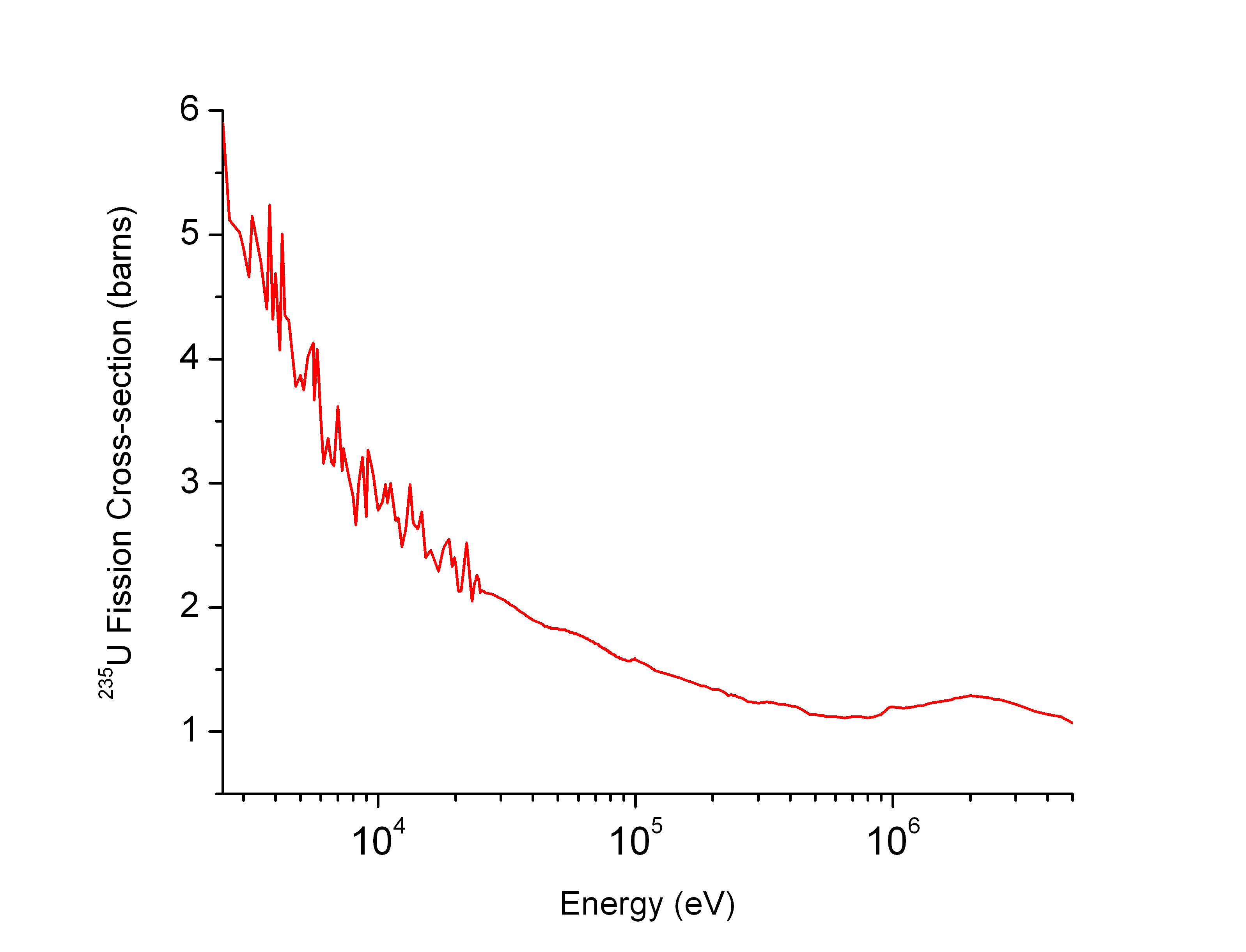
As an example, the plot on the right shows that the fission cross section of
For a given target and reaction, the cross section is strongly dependent on the neutron speed. In the extreme case, the cross section can be, at low energies, either zero (the energy for which the cross section becomes significant is called threshold energy) or much larger than at high energies.
Therefore, a cross section should be defined either at a given energy or should be averaged in an energy range (or group).
As an example, the plot on the right shows that the fission cross section of
Equation 38
 Imagine a spherical target (shown as the dashed grey and red circle in the figure) and a beam of particles (in blue) "flying" at speed ''v'' (vector in blue) in the direction of the target. We want to know how many particles impact it during time interval d''t''. To achieve it, the particles have to be in the green cylinder in the figure (volume ''V''). The base of the cylinder is the geometrical cross section of the target perpendicular to the beam (surface ''σ'' in red) and its height the length travelled by the particles during d''t'' (length ''v'' d''t''):
:
Noting ''n'' the number of particles per unit volume, there are ''n V'' particles in the volume ''V'', which will, per definition of ''V'', undergo a reaction. Noting ''r'' the
Imagine a spherical target (shown as the dashed grey and red circle in the figure) and a beam of particles (in blue) "flying" at speed ''v'' (vector in blue) in the direction of the target. We want to know how many particles impact it during time interval d''t''. To achieve it, the particles have to be in the green cylinder in the figure (volume ''V''). The base of the cylinder is the geometrical cross section of the target perpendicular to the beam (surface ''σ'' in red) and its height the length travelled by the particles during d''t'' (length ''v'' d''t''):
:
Noting ''n'' the number of particles per unit volume, there are ''n V'' particles in the volume ''V'', which will, per definition of ''V'', undergo a reaction. Noting ''r'' the
 In the following, some cross sections which are of importance in a nuclear reactor are given. The thermal cross-section is averaged using a Maxwellian spectrum and the fast cross section is averaged using the uranium-235 fission spectrum. The cross sections are taken from the JEFF-3.1.1 library using JANIS software.JANIS software, https://www.oecd-nea.org/janis/
In the following, some cross sections which are of importance in a nuclear reactor are given. The thermal cross-section is averaged using a Maxwellian spectrum and the fast cross section is averaged using the uranium-235 fission spectrum. The cross sections are taken from the JEFF-3.1.1 library using JANIS software.JANIS software, https://www.oecd-nea.org/janis/
* ''negligible, less than 0.1% of the total cross section and below the Bragg scattering cutoff''
XSPlot an online nuclear cross section plotterNeutron scattering lengths and cross-sections
nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies t ...
, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travellin ...
, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant
A nuclear power plant (NPP) is a thermal power station in which the heat source is a nuclear reactor. As is typical of thermal power stations, heat is used to generate steam that drives a steam turbine connected to a generator that produces ...
. The standard unit for measuring the cross section is the barn
A barn is an agricultural building usually on farms and used for various purposes. In North America, a barn refers to structures that house livestock, including cattle and horses, as well as equipment and fodder, and often grain.Alle ...
, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus.
An isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
(or nuclide
A nuclide (or nucleide, from atomic nucleus, nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was co ...
) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron absorbers and will have a ''capture cross section'' for that reaction. Isotopes that undergo fission are fissionable fuels and have a corresponding ''fission cross section''. The remaining isotopes will simply scatter the neutron, and have a ''scatter cross section''. Some isotopes, like uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However ...
, have nonzero cross sections of all three.
Isotopes which have a large scatter cross section and a low mass are good neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely m ...
s (see chart below). Nuclides which have a large absorption cross section are neutron poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable eff ...
s if they are neither fissile nor undergo decay. A poison that is purposely inserted into a nuclear reactor for controlling its reactivity in the long term and improve its shutdown margin is called a ''burnable'' poison.
Parameters of interest
The neutron cross section, and therefore the probability of an neutron-nucleus interaction, depends on: * the target type (hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
, uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
…),
* the type of nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformatio ...
(scattering, fission…).
* the incident particle energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of ...
, also called speed or temperature (thermal, fast…),
and, to a lesser extent, of:
* its relative angle between the incident neutron and the target nuclide,
* the target nuclide temperature.
Target type dependence
The neutron cross section is defined for a given type of target particle. For example, the capture cross section of deuterium 2H is much smaller than that of common hydrogen 1H. This is the reason why some reactors use heavy water (in which most of the hydrogen is deuterium) instead of ordinary light water as moderator: fewer neutrons are lost by capture inside the medium, hence enabling the use ofnatural uranium
Natural uranium (NU or Unat) refers to uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes ...
instead of enriched uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U ...
. This is the principle of a CANDU reactor.
Type of reaction dependence
The likelihood of interaction between an incident neutron and a target nuclide, independent of the type of reaction, is expressed with the help of the total cross section ''σ''T. However, it may be useful to know if the incoming particle bounces off the target (and therefore continue travelling after the interaction) or disappears after the reaction. For that reason, the scattering and absorption cross sections ''σ''S and ''σ''A are defined and the total cross section is simply the sum of the two partial cross sections: :Absorption cross section
If the neutron is absorbed when approaching the nuclide, the atomic nucleus moves up on the table of isotopes by one position. For instance, 235U becomes 236*U with the * indicating the nucleus is highly energized. This energy has to be released and the release can take place through any of several mechanisms. # The simplest way for the release to occur is for the neutron to be ejected by the nucleus. If the neutron is emitted immediately, it acts the same as in other scattering events. # The nucleus may emit gamma radiation. # The nucleus may β− decay, where a neutron is converted into a proton, an electron and an electron-type antineutrino (the antiparticle of the neutrino) # About 81% of the 236*U nuclei are so energized that they undergo fission, releasing the energy as kinetic motion of the fission fragments, also emitting between one and five free neutrons. * Nuclei that undergo fission as their predominant decay method after neutron capture include 233U, 235U, 237U, 239Pu, 241Pu. * Nuclei that predominantly absorb neutrons and then emit beta particle radiation lead to these isotopes, e.g., 232Th absorbs a neutron and becomes 233*Th, which beta decays to become 233Pa, which in turn beta decays to become 233U. * Isotopes that undergo beta decay transmute from one element to another element. Those that undergo gamma or X-ray emission do not cause a change in element or isotope.Scattering cross-section
The scattering cross-section can be further subdivided into coherentscattering
Scattering is a term used in physics to describe a wide range of physical processes where moving particles or radiation of some form, such as light or sound, are forced to deviate from a straight trajectory by localized non-uniformities (including ...
and incoherent scattering, which is caused by the spin
Spin or spinning most often refers to:
* Spinning (textiles), the creation of yarn or thread by twisting fibers together, traditionally by hand spinning
* Spin, the rotation of an object around a central axis
* Spin (propaganda), an intentionally ...
dependence of the scattering cross-section and, for a natural sample, presence of different isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s of the same element in the sample.
Because neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s interact with the nuclear potential
The nuclear force (or nucleon–nucleon interaction, residual strong force, or, historically, strong nuclear force) is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nucl ...
, the scattering cross-section varies for different isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s of the element in question. A very prominent example is hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
and its isotope deuterium
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one ...
. The total cross-section for hydrogen is over 10 times that of deuterium, mostly due to the large incoherent scattering length The scattering length in quantum mechanics describes low-energy scattering. For potentials that decay faster than 1/r^3 as r\to \infty, it is defined as the following low-energy limit (mathematics), limit:
:
\lim_ k\cot\delta(k) =- \frac\;,
wher ...
of hydrogen. Some metals are rather transparent to neutrons, aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
and zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
being the two best examples of this.
Incident particle energy dependence
 For a given target and reaction, the cross section is strongly dependent on the neutron speed. In the extreme case, the cross section can be, at low energies, either zero (the energy for which the cross section becomes significant is called threshold energy) or much larger than at high energies.
Therefore, a cross section should be defined either at a given energy or should be averaged in an energy range (or group).
As an example, the plot on the right shows that the fission cross section of
For a given target and reaction, the cross section is strongly dependent on the neutron speed. In the extreme case, the cross section can be, at low energies, either zero (the energy for which the cross section becomes significant is called threshold energy) or much larger than at high energies.
Therefore, a cross section should be defined either at a given energy or should be averaged in an energy range (or group).
As an example, the plot on the right shows that the fission cross section of uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exi ...
is low at high neutron energies but becomes higher at low energies. Such physical constraints explain why most operational nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from ...
use a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely m ...
to reduce the energy of the neutron and thus increase the probability of fission which is essential to produce energy and sustain the chain reaction
A chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive feedback leads to a self-amplifying chain of events.
Chain reactions are one way that sys ...
.
A simple estimation of energy dependence of any kind of cross section is provided by the Ramsauer Model,R. W. Bauer, J. D. Anderson, S. M. Grimes, V. A. Madsen, Application of Simple Ramsauer Model to Neutron Total Cross Sections, https://www.osti.gov/bridge/servlets/purl/641282-MK9s2L/webviewable/641282.pdf which is based on the idea that the ''effective'' size of a neutron is proportional to the breadth of the probability density function
In probability theory, a probability density function (PDF), or density of a continuous random variable, is a function whose value at any given sample (or point) in the sample space (the set of possible values taken by the random variable) ca ...
of where the neutron is likely to be, which itself is proportional to the neutron's thermal de Broglie wavelength.
:
Taking as the effective radius of the neutron, we can estimate the area of the circle in which neutrons hit the nuclei of effective radius as
:
While the assumptions of this model are naive, it explains at least qualitatively the typical measured energy dependence of the neutron absorption cross section. For neutrons of wavelength much larger than typical radius of atomic nuclei (1–10 fm, E = 10–1000 keV) can be neglected. For these low energy neutrons (such as thermal neutrons) the cross section is inversely proportional to neutron velocity.
This explains the advantage of using a neutron moderator
In nuclear engineering, a neutron moderator is a medium that reduces the speed of fast neutrons, ideally without capturing any, leaving them as thermal neutrons with only minimal (thermal) kinetic energy. These thermal neutrons are immensely m ...
in fission nuclear reactors. On the other hand, for very high energy neutrons (over 1 MeV), can be neglected, and the neutron cross section is approximately constant, determined just by the cross section of atomic nuclei.
However, this simple model does not take into account so called neutron resonances, which strongly modify the neutron cross section in the energy range of 1 eV–10 keV, nor the threshold energy of some nuclear reactions.
Target temperature dependence
Cross sections are usually measured at 20 °C. To account for the dependence with temperature of the medium (viz. the target), the following formula is used:DOE Fundamentals Handbook, Nuclear Physics and Reactor Theory, DOE-HDBK-1019/1-93 . : where ''σ'' is the cross section at temperature ''T'', and ''σ''0 the cross section at temperature ''T''0 (''T'' and ''T''0 inkelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and ...
s).
The energy is defined at the most likely energy and velocity of the neutron. The neutron population consists of a Maxwellian distribution, and hence the mean energy and velocity will be higher. Consequently also a Maxwellian correction-term √π has to be included when calculating the cross-sectioEquation 38
Doppler broadening
The Doppler broadening of neutron resonances is a very important phenomenon and improvesnuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
stability. The prompt temperature coefficient of most thermal reactors is negative, owing to the nuclear Doppler effect
The Doppler effect or Doppler shift (or simply Doppler, when in context) is the change in frequency of a wave in relation to an observer who is moving relative to the wave source. It is named after the Austrian physicist Christian Doppler, who ...
. Nuclei are located in atoms which are themselves in continual motion owing to their thermal energy (temperature). As a result of these thermal motions, neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s impinging on a target appears to the nuclei in the target to have a continuous spread in energy. This, in turn, has an effect on the observed shape of resonance. The resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscil ...
becomes shorter and wider than when the nuclei are at rest.
Although the shape of resonances changes with temperature, the total area under the resonance remains essentially constant. But this does not imply constant neutron absorption. Despite the constant area under resonance a resonance integral, which determines the absorption, increases with increasing target temperature. This, of course, decreases coefficient k (negative reactivity is inserted).
Link to reaction rate and interpretation
reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per uni ...
onto one target, it gives:
:
It follows directly from the definition of the neutron flux
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travellin ...
:
:
Assuming that there is not one but ''N'' targets per unit volume, the reaction rate ''R'' per unit volume is:
:
Knowing that the typical nuclear radius ''r'' is of the order of 10−12 cm, the expected nuclear cross section is of the order of ''π r''2 or roughly 10−24 cm2 (thus justifying the definition of the barn
A barn is an agricultural building usually on farms and used for various purposes. In North America, a barn refers to structures that house livestock, including cattle and horses, as well as equipment and fodder, and often grain.Alle ...
). However, if measured experimentally ( ''σ'' = ''R'' / (''Φ N'') ), the experimental cross sections vary enormously. As an example, for slow neutrons absorbed by the (n, γ) reaction the cross section in some cases (xenon-135
Xenon-135 (135Xe) is an unstable isotope of xenon with a half-life of about 9.2 hours. 135Xe is a fission product of uranium and it is the most powerful known neutron-absorbing nuclear poison (2 million barns; up to 3 million barns under reac ...
) is as much as 2,650,000 barns, while the cross sections for transmutations by gamma-ray absorption are in the neighborhood of 0.001 barn ( has more examples).
The so-called ''nuclear cross section'' is consequently a purely conceptual quantity representing how big the nucleus should be to be consistent with this simple mechanical model.
Continuous versus average cross section
Cross sections depend strongly on the incoming particle speed. In the case of a beam with multiple particle speeds, the reaction rate ''R'' is integrated over the whole range of energy: : Where ''σ''(''E'') is the continuous cross section, ''Φ''(''E'') the differential flux and ''N'' the target atom density. In order to obtain a formulation equivalent to the mono energetic case, an average cross section is defined: : Where is the integral flux. Using the definition of the integral flux ''Φ'' and the average cross section ''σ'', the same formulation asbefore
Before is the opposite of after, and may refer to:
* ''Before'' (Gold Panda EP), 2009
* ''Before'' (James Blake EP), 2020
* "Before" (song), a 1996 song by the Pet Shop Boys
* "Before", a song by the Empire of the Sun from ''Two Vines''
* "Befo ...
is found:
:
Microscopic versus macroscopic cross section
Up to now, the cross section referred to in this article corresponds to the microscopic cross section ''σ''. However, it is possible to define the macroscopic cross section ''Σ'' which corresponds to the total "equivalent area" of all target particles per unit volume: : where ''N'' is the atomic density of the target. Therefore, since the cross section can be expressed in cm2 and the density in cm−3, the macroscopic cross section is usually expressed in cm−1. Using the equation derived above, the reaction rate ''R'' can be derived using only the neutron flux ''Φ'' and the macroscopic cross section ''Σ'': :Mean free path
Themean free path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as ...
''λ'' of a random particle is the average length between two interactions. The total length ''L'' that non perturbed particles travel during a time interval ''dt'' in a volume ''dV'' is simply the product of the length ''l'' covered by each particle during this time with the number of particles ''N'' in this volume:
:
Noting ''v'' the speed of the particles and ''n'' is the number of particles per unit volume:
:
It follows:
:
Using the definition of the neutron flux
The neutron flux, φ, is a scalar quantity used in nuclear physics and nuclear reactor physics. It is the total length travelled by all free neutrons per unit time and volume. Equivalently, it can be defined as the number of neutrons travellin ...
''Φ''
:
It follows:
:
This average length ''L'' is however valid only for unperturbed particles. To account for the interactions, ''L'' is divided by the total number of reactions ''R'' to obtain the average length between each collision ''λ'':
:
From :
:
It follows:
:
where ''λ'' is the mean free path and ''Σ'' is the macroscopic cross section.
Within stars
Because 8Li and 12Be form natural stopping points on the table of isotopes forhydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-to ...
fusion, it is believed that all of the higher elements are formed in very hot stars where higher orders of fusion predominate. A star like the Sun produces energy
In physics, energy (from Ancient Greek: ἐνέργεια, ''enérgeia'', “activity”) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of ...
by the fusion of simple 1H into 4He through a series of reactions. It is believed that when the inner core exhausts its 1H fuel, the Sun will contract, slightly increasing its core temperature until 4He can fuse and become the main fuel supply. Pure 4He fusion leads to 8Be, which decays back to 2 4He; therefore the 4He must fuse with isotopes either more or less massive than itself to result in an energy producing reaction. When 4He fuses with 2H or 3H, it forms stable isotopes 6Li and 7Li respectively. The higher order isotopes between 8Li and 12C are synthesized by similar reactions between hydrogen, helium, and lithium isotopes.
Typical cross sections
External links
XSPlot an online nuclear cross section plotter
References
{{Reflist cross section Nuclear physics