McLeod gauge on:
[Wikipedia]
[Google]
[Amazon]
 A McLeod gauge is a scientific instrument used to measure very low
A McLeod gauge is a scientific instrument used to measure very low 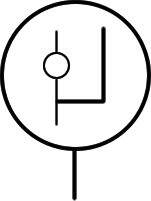 McLeod gauges operate by taking in a sample volume of gas from a vacuum chamber, then compressing it by tilting and infilling with mercury. The pressure in this smaller volume is then measured by a mercury manometer, and knowing the compression ratio (the ratio of the initial and final volumes), the pressure of the original vacuum can be determined by applying
McLeod gauges operate by taking in a sample volume of gas from a vacuum chamber, then compressing it by tilting and infilling with mercury. The pressure in this smaller volume is then measured by a mercury manometer, and knowing the compression ratio (the ratio of the initial and final volumes), the pressure of the original vacuum can be determined by applying
 A McLeod gauge is a scientific instrument used to measure very low
A McLeod gauge is a scientific instrument used to measure very low pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and e ...
s, down to 10−6 Torr
The torr (symbol: Torr) is a unit of pressure based on an absolute scale, defined as exactly of a standard atmosphere (). Thus one torr is exactly (≈ ).
Historically, one torr was intended to be the same as one " millimeter of merc ...
(1.33 m Pa). It was invented in 1874 by Herbert McLeod
Herbert McLeod, FRS (February 1841October 1923) was a English chemist, noted for the invention of the McLeod gauge and for the invention of a sunshine recorder.
Biography
McLeod was born in Stoke Newington on 9 Feb 1841 and died 3 October 1923 ...
(1841–1923). McLeod gauges were once commonly found attached to equipment that operates under vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or " void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often di ...
, such as a lyophilizer. Today, however, these gauges have largely been replaced by electronic vacuum gauges.
The design of a McLeod gauge is somewhat similar to that of a mercury-column manometer. Typically it is filled with mercury. If used incorrectly, this mercury can escape and contaminate the vacuum system attached to the gauge.
 McLeod gauges operate by taking in a sample volume of gas from a vacuum chamber, then compressing it by tilting and infilling with mercury. The pressure in this smaller volume is then measured by a mercury manometer, and knowing the compression ratio (the ratio of the initial and final volumes), the pressure of the original vacuum can be determined by applying
McLeod gauges operate by taking in a sample volume of gas from a vacuum chamber, then compressing it by tilting and infilling with mercury. The pressure in this smaller volume is then measured by a mercury manometer, and knowing the compression ratio (the ratio of the initial and final volumes), the pressure of the original vacuum can be determined by applying Boyle's law
Boyle's law, also referred to as the Boyle–Mariotte law, or Mariotte's law (especially in France), is an experimental gas law that describes the relationship between pressure and volume of a confined gas. Boyle's law has been stated as:
The ...
.
This method is fairly accurate for non-condensable gases, such as oxygen and nitrogen. However, condensable gases, such as water vapour, ammonia, carbon dioxide, and pump-oil vapors may be in gaseous form in the low pressure of the vacuum chamber, but will condense when compressed by the McLeod gauge. The result is an erroneous reading, showing a pressure much lower than actually present. A cold trap
In vacuum applications, a cold trap is a device that condenses all vapors except the permanent gases into a liquid or solid. The most common objective is to prevent vapors being evacuated from an experiment from entering a vacuum pump where they ...
may be used in conjunction with a McLeod gauge to condense these vapors before they enter the gauge.
The McLeod gauge has the advantage that its calibration is nearly the same for all non-condensable gases. The device can be manually operated and the scale read visually, or the process can be automated in various ways. For example, a small electric motor can periodically rotate the assembly to collect a gas sample. If a fine platinum wire is in the capillary tube, its resistance indicates the height of the mercury column around it.
Modern electronic vacuum gauge {{Cat main, Vacuum system
Vacuum
Systems
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its environment, is described by its boun ...
s are simpler to use, less fragile, and do not present a mercury hazard, but their reading is highly dependent on the chemical nature of the gas being measured, and their calibration is unstable. For this reason, McLeod gauges continue to be used as a calibration standard for electronic gauges.
See also
*Penning gauge
Pressure measurement is the measurement of an applied force by a fluid (liquid or gas) on a surface. Pressure is typically measured in units of force per unit of surface area. Many techniques have been developed for the measurement of pressure ...
* Pirani gauge
The Pirani gauge is a robust thermal conductivity gauge used for the measurement of the pressures in vacuum systems. It was invented in 1906 by Marcello Pirani.
Marcello Stefano Pirani was a German physicist working for Siemens & Halske which was ...
References
External links
* http://physics.kenyon.edu/EarlyApparatus/Pneumatics/McLeod_Gauge/McLeod_Gauge.html * https://web.archive.org/web/20060504124236/http://www.tau.ac.il/~phchlab/experiments/vacuum/Techniques_of_high_vacuum/Vacuum5.html Vacuum gauges Pressure gauges Laboratory equipment Laboratory glassware {{Physics-stub