Metal-centered Cycloaddition Reactions on:
[Wikipedia]
[Google]
[Amazon]
A metal-centered cycloaddition is a subtype of the more general class of cycloaddition reactions. In such reactions "two or more unsaturated molecules unite directly to form a ring", incorporating a metal bonded to one or more of the molecules. Cycloadditions involving metal centers are a staple of
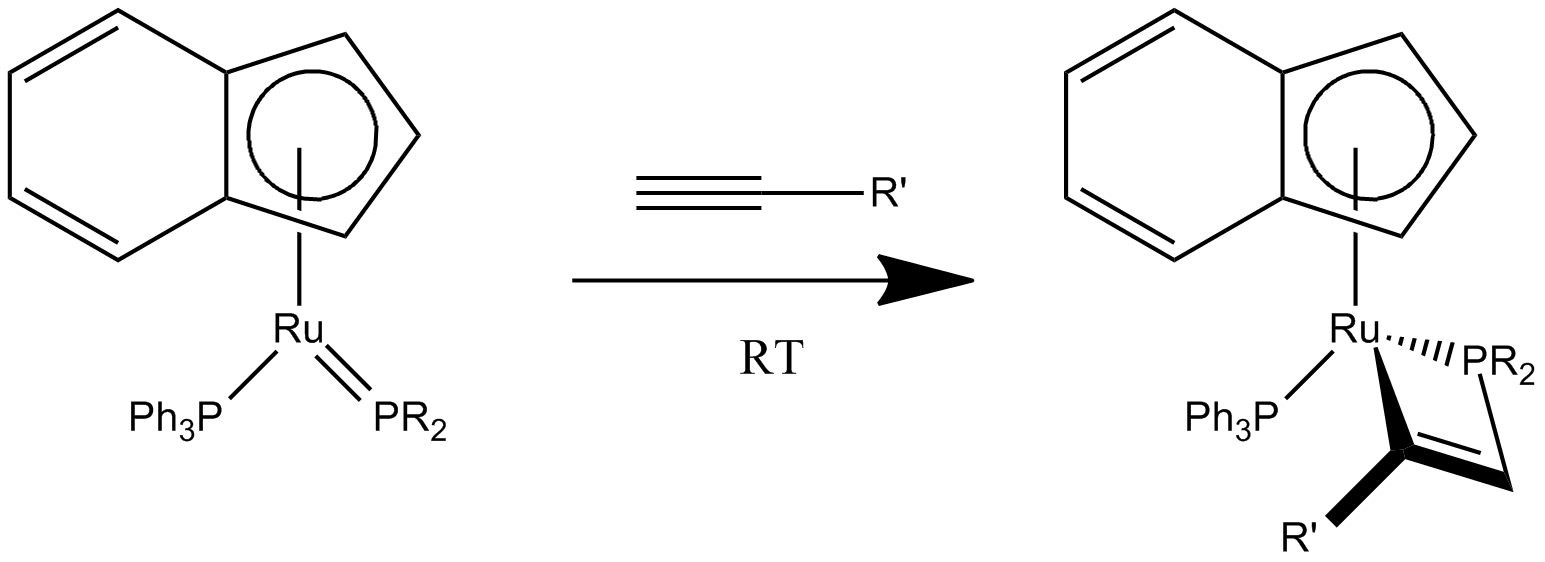



organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product o ...
and organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and ...
chemistry, and are involved in many industrially-valuable synthetic processes.
There are two general types of metal-centered cycloaddition reactions: those in which the metal is incorporated into the cycle (a metallocycle
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates ...
), and those in which the metal is external to the cycle. These can be further divided into "true" cycloadditions (those that take place in a concerted
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend n ...
fashion), and formal cycloadditions (those that take place in a stepwise fashion). Beyond that, they are classified by the number of atoms contributed to the cycle by each of the participants.
For example, olefin metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creat ...
using a Grubbs catalyst
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been devel ...
typically involves a reversible +2cycloaddition. A Ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemic ...
alkylidene
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been rep ...
and an alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
(or alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no ...
) react to form a metallocycle
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates ...
.
Roles of metals in cycloaddition reactions
Conformational control
A common role for a metal centre in cycloaddition reactions is to exert control over theconformation
Conformation generally means structural arrangement and may refer to:
* Conformational isomerism, a form of stereoisomerism in chemistry
** Carbohydrate conformation
** Cyclohexane conformation
** Protein conformation
** Conformation activity rela ...
of the reactants. Metal ions are frequently a component of 1,3-dipolar cycloadditions, and Diels-Alder reactions. A Lewis acidic can coerce a Diene into the reactive cisoid conformation, thereby catalyzing the reaction the Diels-Alder reaction.
A crucial role of the metal in many cycloadditions reactions is to bind simultaneously to the reactants. This brings them into close proximity and encourages them to cyclize. The ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s associated with the metal can direct the approach of the reactants, providing control over regiochemistry
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong ba ...
and stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereo ...
.
Stabilization of reactive species
Cycloadditions that require unstablesynthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 198 ...
s such as carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3CH ...
s or carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" may ...
s are often possible using organometallic compounds. Several synthetic routes to cyclopropyl
A cyclopropyl group is a chemical structure derived from cyclopropane, and can participate in organic reactions that constitute cycloadditions and rearrangement organic reactions of cyclopropane. The group has an empirical formula of C3H5 and c ...
and cyclopropenyl
Cyclopropene is an organic compound with the formula . It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental ...
compounds involve the cycloaddition of a metal carbene
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been re ...
to an alkene or alkyne. Metal-stabilized allyl
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, ...
and pentadienyl complexes are used in +3and +2cycloadditions for preparing seven-membered rings.
Metallocycles
Alkylidene
A transition metal carbene complex is an organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been rep ...
s and other carbene analogs Carbene analogs in chemistry are carbenes with the carbon atom replaced by another chemical element. Just as regular carbenes they appear in chemical reactions as reactive intermediates and with special precautions they can be stabilized and isolate ...
participate readily in cycloaddition reactions. Cycloaddition reactions of Ruthenium phosphinidene
Phosphinidenes (IUPAC: phosphanylidenes, formerly phosphinediyls) are low-valent phosphorus compounds analogous to carbenes and nitrenes, having the general structure RP. The "free" form of these compounds is conventionally described as having a si ...
s with alkenes and alkynes is an active area research and has promise as catalytic cycle for hydrophosphination
Hydrophosphination is the insertion of a carbon-carbon multiple bond into a phosphorus-hydrogen bond forming a new phosphorus-carbon bond. Like other hydrofunctionalizations, the rate and regiochemistry of the insertion reaction is influenced by ...
.
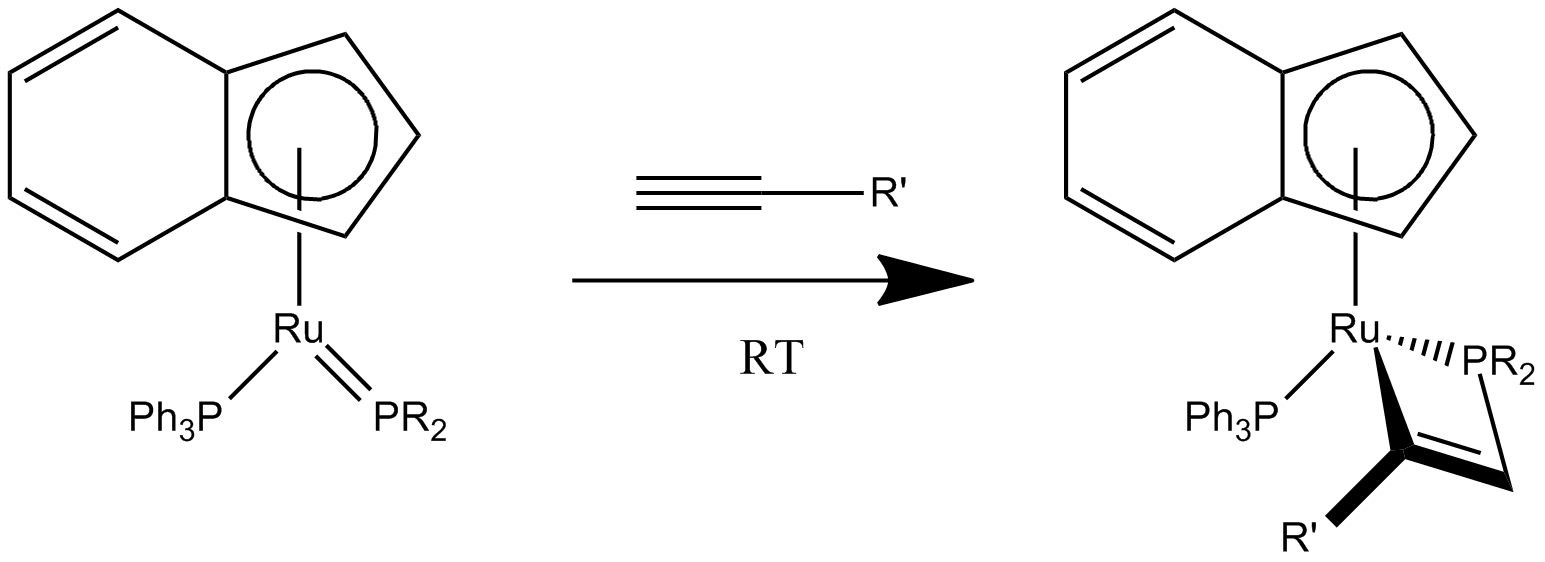
Molecular orbital explanation
Underlying any attempt to explain cycloaddition reactions isFrontier Molecular Orbital Theory In chemistry, frontier molecular orbital theory is an application of MO theory describing HOMO/LUMO interactions.
History
In 1952, Kenichi Fukui published a paper in the ''Journal of Chemical Physics'' titled "A molecular theory of reactivity ...
, which describes the interaction between the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO) of the reactants. A cycloaddition will only proceed if the HOMO and LUMO have an allowed symmetry and are similar in energy. Metals play a crucial role in cycloaddition reactions because they can bind to unsaturated molecules, changing the symmetries and energy levels of the HOMO and/or LUMO. The Woodward-Hoffmann rules and Green-Davies-Mingos rules can provide some indication of the effects of metal-bonding on cycloaddition reactions.
As an example, free Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
is extremely unreactive in cycloadditions due to its aromaticity
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
. Coordination of Benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
to a highly reduced Tricarbonylmanganese centre allows the Benzene to undergo cycloaddition with Diphenylketene
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl kete ...
.

Examples
+2cycloaddition of two alkynes
Althoughcyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is ...
s can only exist briefly in the free state, they can exist indefinitely as metal ligands. They can be formed as ligands in-situ by the +2cycloaddition of sterically bulky alkynes bound to a metal.

Benzannulation
The Dötz reaction is a formal +2+1cycloaddition of two alkynes, a carbene, and a carbonyl ligand to form a benzene ring.Formal +4cycloaddition
An unusual formal +4cycloaddition was reported by Kreiter et al.{{cite journal, last=Kreiter, first=Cornelius G, author2=Lehr, Klaus, title=Photochemische Reaktionen von Übergangsmetall-Organyl-Komplexen mit Olefinen, journal=Journal of Organometallic Chemistry, volume=406, issue=1–2, pages=159–170, doi=10.1016/0022-328X(91)83183-5, year=1991 Nine-membered rings are unusual and only a handful of synthetic routes to rings of this size are known.
See also
*Cycloaddition reaction
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". Th ...
*Frontier Molecular Orbital Theory In chemistry, frontier molecular orbital theory is an application of MO theory describing HOMO/LUMO interactions.
History
In 1952, Kenichi Fukui published a paper in the ''Journal of Chemical Physics'' titled "A molecular theory of reactivity ...
* Organometallic chemistry
*Pericyclic reaction
In organic chemistry, a pericyclic reaction is the type of organic reaction wherein the transition state of the molecule has a cyclic geometry, the reaction progresses in a concerted fashion, and the bond orbitals involved in the reaction overlap ...
* 1,3-Dipolar cycloaddition
* Diels-Alder reaction
References