Malate–aspartate Shuttle on:
[Wikipedia]
[Google]
[Amazon]
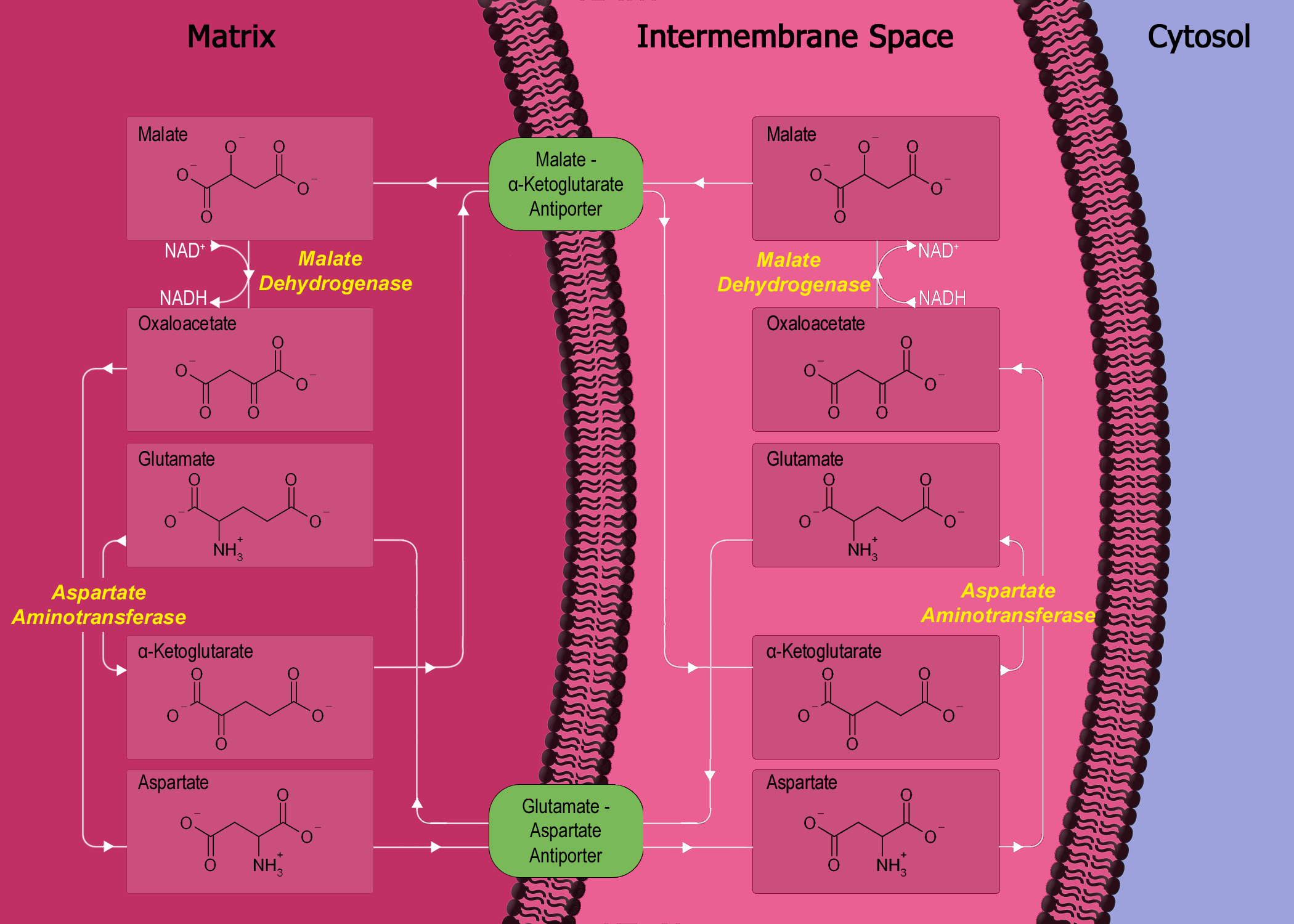 The malate–aspartate shuttle (sometimes simply the malate shuttle) is a biochemical system for translocating electrons produced during
The malate–aspartate shuttle (sometimes simply the malate shuttle) is a biochemical system for translocating electrons produced during
 The malate–aspartate shuttle (sometimes simply the malate shuttle) is a biochemical system for translocating electrons produced during
The malate–aspartate shuttle (sometimes simply the malate shuttle) is a biochemical system for translocating electrons produced during glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvic acid, pyruvate and, in most organisms, occurs in the liquid part of cells (the cytosol). The Thermodynamic free energy, free energy released in this process is used to form ...
across the semipermeable inner membrane of the mitochondrion
The inner mitochondrial membrane (IMM) is the mitochondrial membrane which separates the mitochondrial matrix from the intermembrane space.
Structure
The structure of the inner mitochondrial membrane is extensively folded and compartmentalized. T ...
for oxidative phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order ...
in eukaryote
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
s. These electrons enter the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
of the mitochondria via reduction equivalents to generate ATP. The shuttle system is required because the mitochondrial inner membrane is impermeable to NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an ade ...
, the primary reducing equivalent of the electron transport chain. To circumvent this, malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms ( ...
carries the reducing equivalents
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox reacti ...
across the membrane.
Components
The shuttle consists of four protein parts: *malate dehydrogenase
Malate dehydrogenase () (MDH) is an enzyme that reversibly catalyzes the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH. This reaction is part of many metabolic pathways, including the citric acid cycle. Other malate ...
in the mitochondrial matrix and intermembrane space.
*aspartate aminotransferase
Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase (GOT, SGOT), is a pyridoxal phosphate (PLP)-dependent transaminase enzyme () that was first described by Arthur ...
in the mitochondrial matrix and intermembrane space.
* malate-alpha-ketoglutarate antiporter in the inner membrane.
* glutamate-aspartate antiporter in the inner membrane.
Mechanism
The primaryenzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
in the malate–aspartate shuttle is malate dehydrogenase. Malate dehydrogenase is present in two forms in the shuttle system: mitochondrial malate dehydrogenase and cytosolic malate dehydrogenase. The two malate dehydrogenases are differentiated by their location and structure, and catalyze their reactions in opposite directions in this process.
First, in the cytosol, malate dehydrogenase catalyses the reaction of oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
and NADH to produce malate and NAD+. In this process, two electrons generated from NADH, and an accompanying H+, are attached to oxaloacetate to form malate.
Once malate is formed, the first antiporter (malate- alpha-ketoglutarate) imports the malate from the cytosol into the mitochondrial matrix and also exports alpha-ketoglutarate from the matrix into the cytosol simultaneously. After malate reaches the mitochondrial matrix, it is converted by mitochondrial malate dehydrogenase into oxaloacetate, during which NAD+ is reduced with two electrons to form NADH. Oxaloacetate is then transformed into aspartate (since oxaloacetate cannot be transported into the cytosol) by mitochondrial aspartate aminotransferase. Since aspartate is an amino acid, an amino radical needs to be added to the oxaloacetate. This is supplied by glutamate, which in the process is transformed into alpha-ketoglutarate by the same enzyme.
The second antiporter (AGC1 or AGC2) imports glutamate from the cytosol into the matrix and exports aspartate from the matrix to the cytosol. Once in the cytosol, aspartate is converted by cytosolic aspartate aminotransferase to oxaloacetate.
The net effect of the malate–aspartate shuttle is purely redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
: NADH in the cytosol is oxidized to NAD+, and NAD+ in the matrix is reduced to NADH. The NAD+ in the cytosol can then be reduced again by another round of glycolysis, and the NADH in the matrix can be used to pass electrons to the electron transport chain so ATP can be synthesized.
Since the malate–aspartate shuttle regenerates NADH inside the mitochondrial matrix, it is capable of maximizing the number of ATPs produced in glycolysis (3/NADH), ultimately resulting in a net gain of 38 ATP molecules per molecule of glucose metabolized. Compare this to the glycerol 3-phosphate shuttle, which reduces FAD+ to produce FADH2, donates electrons to the quinone pool in the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
, and is capable of generating only 2 ATPs per NADH generated in glycolysis (ultimately resulting in a net gain of 36 ATPs per glucose metabolized). (These ATP numbers are prechemiosmotic, and should be reduced in light of the work of Mitchell and many others. Each NADH produces only 2.5 ATPs, and each FADH2 produces only 1.5 ATPs. Hence, the ATPs per glucose should be reduced to 32 from 38 and 30 from 36. The extra H+ required to bring in the inorganic phosphate during oxidative-phosphorylation contributes to the 30 and 32 numbers as well).
Regulation
The activity of malate–aspartate shuttle is modulated by arginine methylation of malate dehydrogenase 1 (MDH1). Protein arginine N-methyltransferase CARM1 methylates and inhibits MDH1 by disrupting its dimerization, which represses malate–aspartate shuttle and inhibits mitochondria respiration ofpancreatic cancer
Pancreatic cancer arises when cell (biology), cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a Neoplasm, mass. These cancerous cells have the malignant, ability to invade other parts of ...
cells.
Interactive pathway map
See also
* Glycerol phosphate shuttle * Mitochondrial shuttleReferences
* {{cite book , author1=Monty Krieger , author2=Matthew P Scott , author3=Matsudaira, Paul T. , author4=Lodish, Harvey F. , author5=Darnell, James E. , author6=Lawrence Zipursky , author7=Kaiser, Chris , author8=Arnold Berk , title=Molecular Cell Biology, Fifth Edition , year=2003 , publisher=W. H. Freeman , location=San Francisco , isbn=0-7167-4366-3 , url-access=registration , url=https://archive.org/details/molecularcellbio00harv Biochemical reactions Cellular respiration