Kendrick mass on:
[Wikipedia]
[Google]
[Amazon]
The Kendrick mass is defined by setting the mass of a chosen molecular fragment, typically CH2, to an integer value in amu (
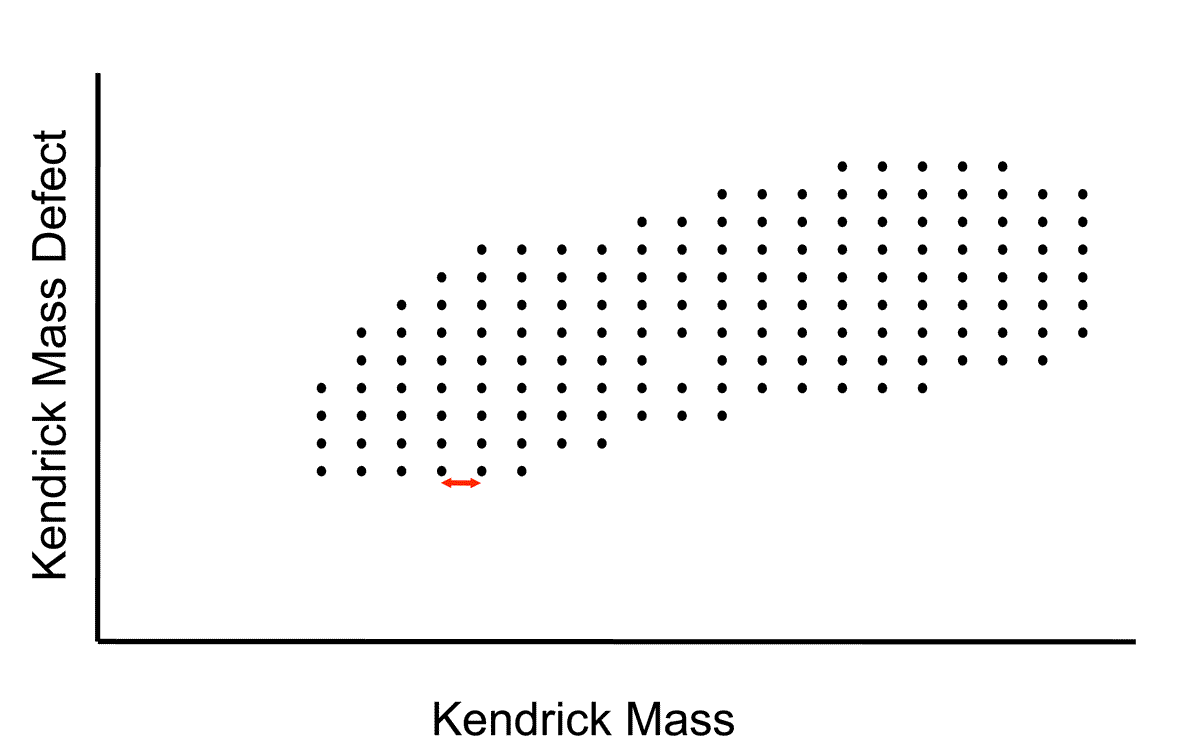 In a Kendrick mass analysis, the Kendrick mass defect is plotted as function of nominal Kendrick mass for ions observed in a mass spectrum. Ions of the same family, for example the members of an alkylation series, have the same Kendrick mass defect but different nominal Kendrick mass and are positioned along a horizontal line on the plot. If the composition of one ion in the family can be determined, the composition of the other ions can be inferred. Horizontal lines of different Kendrick mass defect correspond to ions of different composition, for example degree of saturation or heteroatom content.
A Kendrick mass analysis is often used in conjunction with a
In a Kendrick mass analysis, the Kendrick mass defect is plotted as function of nominal Kendrick mass for ions observed in a mass spectrum. Ions of the same family, for example the members of an alkylation series, have the same Kendrick mass defect but different nominal Kendrick mass and are positioned along a horizontal line on the plot. If the composition of one ion in the family can be determined, the composition of the other ions can be inferred. Horizontal lines of different Kendrick mass defect correspond to ions of different composition, for example degree of saturation or heteroatom content.
A Kendrick mass analysis is often used in conjunction with a
atomic mass units
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at re ...
). It is different from the IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is ...
definition, which is based on setting the mass of 12C isotope to exactly 12 amu. The Kendrick mass is often used to identify homologous compounds differing only by a number of base units in high resolution mass spectra
A mass spectrum is a histogram plot of intensity vs. ''mass-to-charge ratio'' (''m/z'') in a chemical sample, usually acquired using an instrument called a ''mass spectrometer''. Not all mass spectra of a given substance are the same; for example ...
. This definition of mass was first suggested in 1963 by chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties. Chemists carefully describe th ...
Edward Kendrick, and it has been adopted by scientists working in the area of high-resolution mass spectrometry, environmental analysis, proteomics
Proteomics is the large-scale study of proteins. Proteins are vital parts of living organisms, with many functions such as the formation of structural fibers of muscle tissue, enzymatic digestion of food, or synthesis and replication of DNA. I ...
, petroleomics, metabolomics, polymer analysis, etc.
Definition
According to the procedure outlined by Kendrick, the mass of CH2 is defined as exactly 14 Da, instead of the IUPAC mass of 14.01565 Da. To convert an IUPAC mass of a particular compound to the Kendrick mass, the equation : is used. The mass in dalton units (''Da'') can be converted to the Kendrick scale by dividing by 1.0011178. Other groups of atoms in addition to CH2 can be used define the Kendrick mass, for example CO2, H2, H2O, and O. In this case, the Kendrick mass for a family of compounds F is given by :. For the hydrocarbon analysis, F=CH2. As an example, Kendrick analysis has been used for visualizing families of halogenated compounds of environmental interest that differ only by the number of chlorine, bromine or fluorine substitutions. A recent publication has suggested that Kendrick mass be expressed in Kendrick units with symbol ''Ke''.Kendrick mass defect
The Kendrickmass defect
Nuclear binding energy in experimental physics is the minimum energy that is required to disassemble the nucleus of an atom into its constituent protons and neutrons, known collectively as nucleons. The binding energy for stable nuclei is always ...
is defined as the exact Kendrick mass subtracted from the nominal
Nominal may refer to:
Linguistics and grammar
* Nominal (linguistics), one of the parts of speech
* Nominal, the adjectival form of "noun", as in "nominal agreement" (= "noun agreement")
* Nominal sentence, a sentence without a finite verb
* Nou ...
(integer) Kendrick mass:
:
In recent years the equation has changed due to rounding errors to:
:
The members of an alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
series have the same degree of unsaturation
In the analysis of the molecular formula of organic molecules, the degree of unsaturation (also known as the index of hydrogen deficiency (IHD), double bond equivalents, or unsaturation index) is a calculation that determines the total number of r ...
and number of heteroatoms ( nitrogen, oxygen and sulfur) but differ in the number of CH2 units. Members of an alkylation series have the same Kendrick mass defect.
The Kendrick mass defect has also been defined as
: .
The abbreviations ''KM'' and ''KMD'' have been used for Kendrick mass and Kendrick mass defect, respectively.
Kendrick mass analysis
 In a Kendrick mass analysis, the Kendrick mass defect is plotted as function of nominal Kendrick mass for ions observed in a mass spectrum. Ions of the same family, for example the members of an alkylation series, have the same Kendrick mass defect but different nominal Kendrick mass and are positioned along a horizontal line on the plot. If the composition of one ion in the family can be determined, the composition of the other ions can be inferred. Horizontal lines of different Kendrick mass defect correspond to ions of different composition, for example degree of saturation or heteroatom content.
A Kendrick mass analysis is often used in conjunction with a
In a Kendrick mass analysis, the Kendrick mass defect is plotted as function of nominal Kendrick mass for ions observed in a mass spectrum. Ions of the same family, for example the members of an alkylation series, have the same Kendrick mass defect but different nominal Kendrick mass and are positioned along a horizontal line on the plot. If the composition of one ion in the family can be determined, the composition of the other ions can be inferred. Horizontal lines of different Kendrick mass defect correspond to ions of different composition, for example degree of saturation or heteroatom content.
A Kendrick mass analysis is often used in conjunction with a Van Krevelen diagram
Van Krevelen diagrams are graphical plots developed by
Dirk Willem van Krevelen (chemist and professor of fuel technology at the TU Delft) and used to assess the origin and maturity of kerogen and petroleum. The diagram cross-plots the hydrogen: ...
, a two- or three- dimensional graphical analysis in which the elemental composition of the compounds are plotted according to the atomic ratios H/C, O/C, or N/C.
Kendrick mass defect analysis of polymers and alternative base units
Because Kendrick mass defect analysis can be carried out by substituting any repeating unit for CH2, KMD analysis is particularly useful for the visualizing the data from polymer mass spectra. For example, a Kendrick mass defect plot of an ethylene oxide/propylene oxide copolymer can be created by using ethylene oxide (C2H4O) as the base unit and calculating the Kendrick mass as: where 44.02621 is the calculated IUPAC mass for C2H4O. Alternatively, a KMD plot can be constructed for the same copolymer by using propylene oxide as the base unit. Polymer mass spectra containing multiple charge ions exhibit isotopic splitting.Fractional base units and referenced KMD plots
Kendrick mass defect plots created by using fractional base units exhibit enhanced resolution. Referenced Kendrick mass defect plots (KMD plots referenced to the terminal group and adduct composition) with fractional base units can be used to obtain an overview of copolymer composition.See also
* PetroleomicsNotes
{{DEFAULTSORT:Kendrick Mass Units of mass Metrology Mass spectrometry