Jemmis mno rules on:
[Wikipedia]
[Google]
[Amazon]
In
 ''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 2 + 20 + 0 + 0 + 0 = 22 SEPs are required; 16 BH units provide 16 pairs; four shared boron atoms provide 6 pairs, which describes why is stable as a neutral species.
''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 2 + 20 + 0 + 0 + 0 = 22 SEPs are required; 16 BH units provide 16 pairs; four shared boron atoms provide 6 pairs, which describes why is stable as a neutral species.
 According to the ''m'' + ''n'' + ''o'' + ''p'' − ''q'' rule,
According to the ''m'' + ''n'' + ''o'' + ''p'' − ''q'' rule,
 is a bis-''nido'' edge-shared polyhedron. Here, ''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 2 + 18 + 0 + 2 − 0 = 22; 16 BH units provide 16 pairs, 4 bridging hydrogen atoms provide 2 pairs, two shared boron atoms provide 3 pairs, along with the two negative charges which provide 1 pair.
is a bis-''nido'' edge-shared polyhedron. Here, ''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 2 + 18 + 0 + 2 − 0 = 22; 16 BH units provide 16 pairs, 4 bridging hydrogen atoms provide 2 pairs, two shared boron atoms provide 3 pairs, along with the two negative charges which provide 1 pair.
 The structure of β-rhombohedral boron is complicated by the presence of partial occupancies and vacancies. The idealized unit cell, has been shown to be electron-deficient and hence metallic according to theoretical studies, but β-boron is a semiconductor. Application of the Jemmis rule shows that the partial occupancies and vacancies are necessary for electron sufficiency.
can be conceptually divided into a fragment and a () fragment. According to Wade's rule, the fragment requires 8 electrons (the icosahedron at the centre (green) requires 2 electrons; each of the six pentagonal pyramids (black and red) completes an icosahedron in the extended structure; as such the electronic requirement for each of them is 1). The or is formed by the condensation of 6 icosahedra and two
The structure of β-rhombohedral boron is complicated by the presence of partial occupancies and vacancies. The idealized unit cell, has been shown to be electron-deficient and hence metallic according to theoretical studies, but β-boron is a semiconductor. Application of the Jemmis rule shows that the partial occupancies and vacancies are necessary for electron sufficiency.
can be conceptually divided into a fragment and a () fragment. According to Wade's rule, the fragment requires 8 electrons (the icosahedron at the centre (green) requires 2 electrons; each of the six pentagonal pyramids (black and red) completes an icosahedron in the extended structure; as such the electronic requirement for each of them is 1). The or is formed by the condensation of 6 icosahedra and two
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, the Jemmis ''mno'' rules represent a unified rule for predicting and systematizing structures of compounds, usually clusters
may refer to:
Science and technology Astronomy
* Cluster (spacecraft), constellation of four European Space Agency spacecraft
* Cluster II (spacecraft), a European Space Agency mission to study the magnetosphere
* Asteroid cluster, a small ...
. The rules involve electron counting. They were formulated by E. D. Jemmis to explain the structures of condensed polyhedral boranes such as , which are obtained by condensing polyhedral boranes by sharing a triangular face, an edge, a single vertex, or four vertices. These rules are additions and extensions to Wade's rules and polyhedral skeletal electron pair theory
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by ...
. The Jemmis ''mno'' rule provides the relationship between polyhedral boranes, condensed polyhedral boranes, and β-rhombohedral boron. This is similar to the relationship between benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, condensed benzenoid aromatics, and graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
, shown by Hückel's 4''n'' + 2 rule, as well as the relationship between tetracoordinate tetrahedral carbon compounds and diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
. The Jemmis ''mno'' rules reduce to Hückel's rule when restricted to two dimensions and reduce to Wade's rules when restricted to one polyhedron.
Electron-counting rules
Electron-counting rules are used to predict the preferred electron count for molecules. Theoctet rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The ru ...
, the 18-electron rule, and Hückel's 4''n'' + 2 pi-electron rule are proven to be useful in predicting the molecular stability. Wade's rules were formulated to explain the electronic requirement of monopolyhedral borane clusters. The Jemmis ''mno'' rules are an extension of Wade's rules, generalized to include condensed polyhedral boranes as well.
The first condensed polyhedral borane, , is formed by sharing four vertices between two icosahedra. According to Wade's ''n'' + 1 rule for ''n''-vertex '' closo'' structures, should have a charge of +2 (''n'' + 1 = 20 + 1 = 21 pairs required; 16 BH units provide 16 pairs; four shared boron atoms provide 6 pairs; thus 22 pairs are available). To account for the existence of as a neutral species, and to understand the electronic requirement of condensed polyhedral clusters, a new variable, ''m'', was introduced and corresponds to the number of polyhedra (sub-clusters). In Wade's ''n'' + 1 rule, the 1 corresponds to the core bonding molecular orbital (BMO) and the ''n'' corresponds to the number of vertices, which in turn is equal to the number of tangential surface BMOs. If ''m'' polyhedra condense to form a macropolyhedron, ''m'' core BMOs will be formed. Thus the skeletal electron pair (SEP) requirement of closo-condensed polyhedral clusters is ''m'' + ''n''.
Single-vertex sharing is a special case where each subcluster needs to satisfy Wade's rule separately. Let ''a'' and ''b'' be the number of vertices in the subclusters including the shared atom. The first cage requires ''a'' + 1 and the second cage requires ''b'' + 1 SEPs. Therefore, a total of ''a'' + ''b'' + 2 or ''a'' + ''b'' + ''m'' SEPs are required; but ''a'' + ''b'' = ''n'' + 1, as the shared atom is counted twice. The rule can be modified to ''m'' + ''n'' + 1, or generally ''m'' + ''n'' + ''o'', where ''o'' corresponds to the number of single-vertex sharing condensations. The rule can be made more general by introducing a variable, ''p'', corresponding to the number of missing vertices, and ''q'', the number of caps. As such, the generalized Jemmis rule can be stated as follows:
:The SEP requirement of condensed polyhedral clusters is ''m'' + ''n'' + ''o'' + ''p'' − ''q'', where ''m'' is the number of subclusters, ''n'' is the number of vertices, ''o'' is the number of single-vertex shared condensations, ''p'' is the number of missing vertices and ''q'' is the number of caps.
Examples
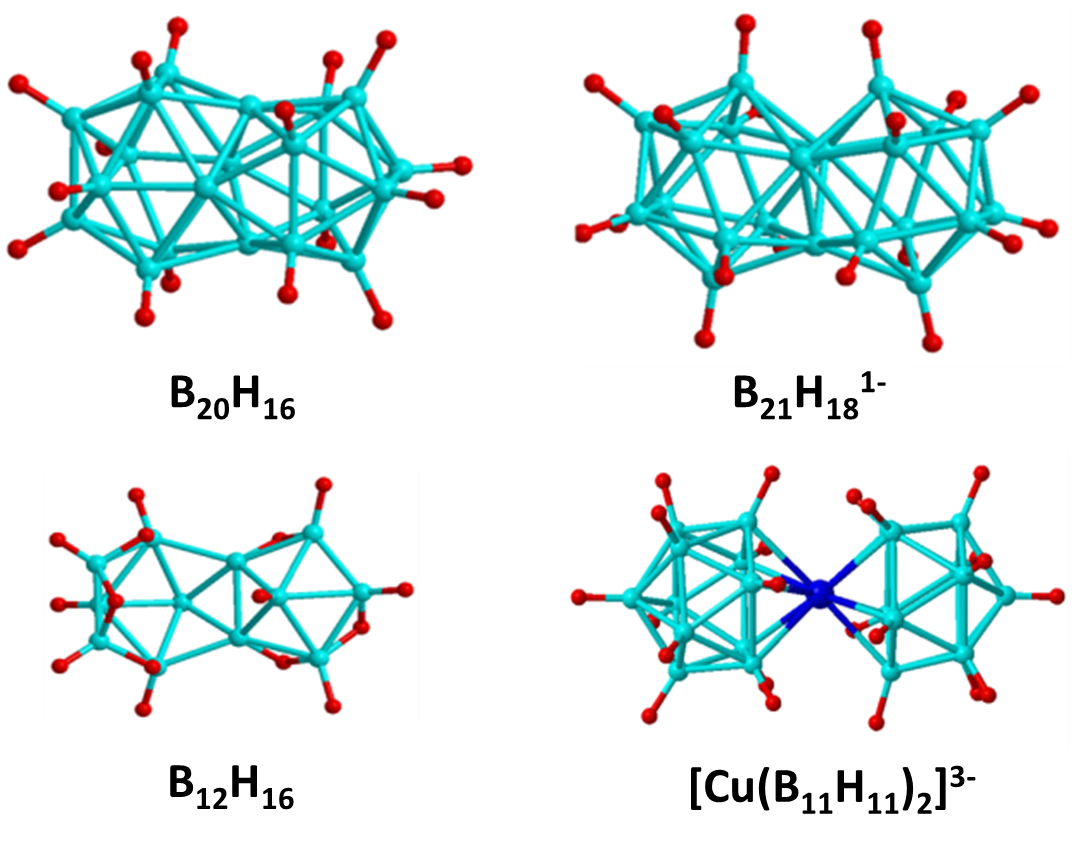
B20H16
 ''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 2 + 20 + 0 + 0 + 0 = 22 SEPs are required; 16 BH units provide 16 pairs; four shared boron atoms provide 6 pairs, which describes why is stable as a neutral species.
''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 2 + 20 + 0 + 0 + 0 = 22 SEPs are required; 16 BH units provide 16 pairs; four shared boron atoms provide 6 pairs, which describes why is stable as a neutral species.
B21H
''closo''- is formed by the face-sharing condensation of two icosahedra. The ''m'' + ''n'' + ''o'' + ''p'' − ''q'' rule demands 23 SEPs; 18 BH units provide 18 pairs and 3 shared boron atoms provide pairs; the negative charge provides one half pair.B12H16
The bis-''nido''- is formed by the edge-sharing condensation of a ''nido''- unit and a ''nido''- unit. The ''m'' + ''n'' + ''o'' + ''p'' − ''q'' count of 16 SEPs are satisfied by ten BH units which provide 10 pairs, two shared boron atoms which provide 3 pairs, and six bridging H atoms which provide 3 pairs.Cu(B11H11)
''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 26 SEPs. A transition metal with ''n'' valence electrons provides ''n'' − 6 electrons for skeletal bonding as 6 electrons occupying the metal-like orbitals do not contribute much to the cluster bonding. Therefore Cu provides pairs, 22 BH units provide 22 pairs; three negative charges provide pairs.Ferrocene
ferrocene
Ferrocene is an organometallic chemistry, organometallic compound with the formula . The molecule is a Cyclopentadienyl complex, complex consisting of two Cyclopentadienyl anion, cyclopentadienyl rings sandwiching a central iron atom. It is an o ...
requires 2 + 11 + 1 + 2 − 0 = 16 SEPs. 10 CH units provide 15 pairs while Fe provides one pair.
B18H
Triple-decker complexes
Triple-decker complexes are known to obey a 30-valence electron (VE) rule. Subtracting 6 pairs of nonbonding electrons from the two metal atoms brings the number of SEPs to 9 pairs. For a triple-decker complex with as the decks, ''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 3 + 17 + 2 + 2 − 0 = 24. Subtracting the 15 pairs corresponding to C–Csigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
s, it becomes 9 pairs. For example, consider : 15 C–CH3 groups provide pairs. Each ruthenium atom provides one pair. Removing the electron corresponding to the positive charge of the complex leads to a total of + 2 − = 24 pairs.
β-Rhombohedral boron
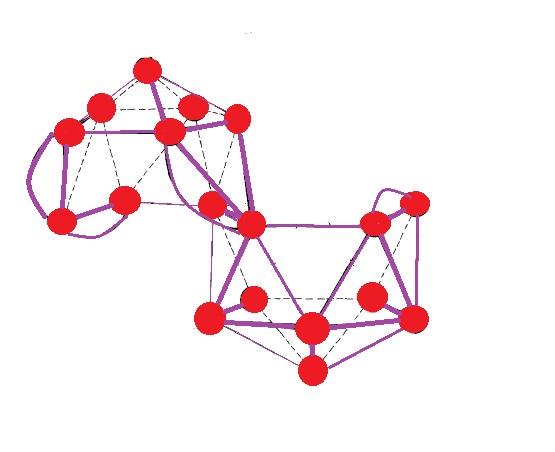
 The structure of β-rhombohedral boron is complicated by the presence of partial occupancies and vacancies. The idealized unit cell, has been shown to be electron-deficient and hence metallic according to theoretical studies, but β-boron is a semiconductor. Application of the Jemmis rule shows that the partial occupancies and vacancies are necessary for electron sufficiency.
can be conceptually divided into a fragment and a () fragment. According to Wade's rule, the fragment requires 8 electrons (the icosahedron at the centre (green) requires 2 electrons; each of the six pentagonal pyramids (black and red) completes an icosahedron in the extended structure; as such the electronic requirement for each of them is 1). The or is formed by the condensation of 6 icosahedra and two
The structure of β-rhombohedral boron is complicated by the presence of partial occupancies and vacancies. The idealized unit cell, has been shown to be electron-deficient and hence metallic according to theoretical studies, but β-boron is a semiconductor. Application of the Jemmis rule shows that the partial occupancies and vacancies are necessary for electron sufficiency.
can be conceptually divided into a fragment and a () fragment. According to Wade's rule, the fragment requires 8 electrons (the icosahedron at the centre (green) requires 2 electrons; each of the six pentagonal pyramids (black and red) completes an icosahedron in the extended structure; as such the electronic requirement for each of them is 1). The or is formed by the condensation of 6 icosahedra and two trigonal bipyramid
A triangular bipyramid is a hexahedron with six triangular faces constructed by attaching two tetrahedra face-to-face. The same shape is also known as a triangular dipyramid or trigonal bipyramid. If these tetrahedra are regular, all faces of a t ...
s. Here, ''m'' + ''n'' + ''o'' + ''p'' − ''q'' = 8 + 57 + 1 + 0 − 0 = 66 pairs required for stability, but are available. Therefore the fragment has 3 excess electrons and the idealized is missing 5 electrons. The 3 excess electrons in the fragment can be removed by removing one B atom, which leads to (). The requirement of 8 electrons by the fragment can be satisfied by boron atoms and the unit cell contains 48 + 56 + = , which is very close to the experimental result.
References
{{Chemical bonds Cluster chemistry Inorganic chemistry